Implications
Experimental comparisons of the nutritional value of essential oils (EO) is often performed under relatively high hygiene status, even though the large-scale broiler producers rear birds in houses with relatively high stocking density and lower hygiene status. Essential oils can influence intestinal microflora, immune responses and animal health, thus their impact may differ between rearing conditions. This information helps to inform the poultry industry of the benefit of using standardised EO combinations for inclusion in broiler chicken feeds, reared under relatively low hygiene status provided by recycled litter.
Introduction
Phytogenic feed additives are plant-derived products (also referred to as EO, phytogenics and phytobiotics) used in animal feeding to improve the performance of agricultural livestock (Windisch et al., 2008). Although the number of scientific publications on phytogenic feed additives significantly increased over the last two decades, the knowledge regarding their modes of action and aspects of application is still rather limited. Most experiments involving plant extracts in poultry have studied separately their impact on production performance (Iskender et al., Reference Iskender, Yenice, Dokumacioglu, Kaynar, Hayirli and Kaya2017), dietary nutrient and energy availability (Bravo et al., Reference Bravo, Pirgozliev and Rose2011 and Reference Bravo, Pirgozliev and Rose2014), intestinal microflora (Altop et al., Reference Altop, Erener, Duru and Isik2017), immune responses (Lee et al., Reference Lee, Lillehoj, Jang, Lee, Park, Bravo and Lillehoj2011) and animal health (Uyar et al., Reference Uyar, Yener and Dogan2016); however, very few of them studied the impact of the rearing conditions on the aforementioned variables.
Research by Pirgozliev et al. (Reference Pirgozliev, Bravo and Rose2014) suggested that the efficiency of dietary EO may be influenced by the hygiene status of poultry houses. Thus, suggesting that the increased levels of normal flora and opportunistic pathogens from the litter flooring may have an impact on the studied variables. Previous studies indicated that certain EO might have beneficial effects on animal performance and health status because of other properties besides their respective functional characteristics (Windisch et al., 2008). A report by Burt (Reference Burt2004) showed that EO, including carvacrol, cinnamaldehyde and capsicum oleoresin, in vitro exhibit antibacterial and antimicrobial effects. Essential oils have also been reported to improve animal performance because of their stimulating effect on pancreatic and intestinal enzyme activity, on bile flow and bile acid secretion or by a direct bactericidal effect on potential pathogen microorganisms of the gut microflora (Hardy, Reference Hardy2002). Moreover, mixtures of spices exhibited an additive effect regarding their pancreatic enzyme stimulation compared with the spices taken individually (Platel et al., Reference Platel, Rao, Saraswathi and Srinivasan2002). In addition, EO supplementation would affect some components of gut health and intestinal barrier, including intestine structure, bacteria populations and microbial metabolites released in the gut lumen (Lee et al., Reference Lee, Lillehoj, Jang, Lee, Park, Bravo and Lillehoj2011; Salami et al., Reference Salami, Guinguina, Agboola, Omede, Agbonlahor and Tayyab2016; Altop et al., Reference Altop, Erener, Duru and Isik2017). Based on this, we hypothesised that beneficial effects of EO are more pronounced under less hygienic housing conditions, for example, microbial loading in the litter.
Therefore, the objectives of the current study were to investigate the effect of a commercial mixture of EO on the performance, available energy, mineral and nutrient utilisation, digestive tract variables, antioxidative status and inflammation when fed to broilers reared on recycled litter.
Material and methods
Diet formulation
Birds were fed one of four diets. There were two control diets based on either wheat (WC) or maize (MC), which were formulated to be iso-energetic (12.13 MJ/kg apparent metabolisable energy (AME)) and iso-nitrogenic (215 g/kg CP) (Table 1). Barley and rye were included in the diet formulation to enhance the detection of differences between treatments, due to their non-starch polysaccharide (NSP) content. The other two diets were the control diets supplemented with a standardised combination of EO (XTRACT 6930; Pancosma S.A., Geneva, Switzerland; thereafter EO) including 5% carvacrol, 3% cinnamaldehyde and 2% capsicum oleoresin (100 g/tonne, respectively, i.e. WC+EO; MC+EO). The EO were added in powder form to the diets and all diets were fed as mash. The diets did not contain any coccidiostat or antimicrobial growth promoters, prophylactic or other similar additives.
Table 1 Composition of the experimental control diets, fed to broiler chickens from 7 to 21 days of age
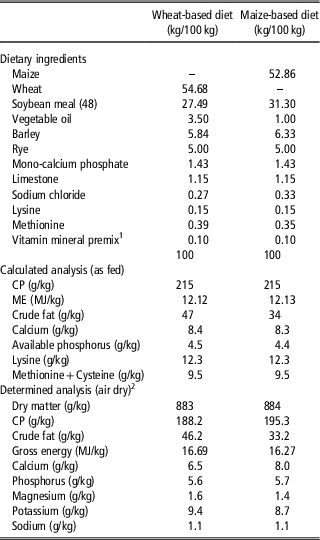
ME=metabolisable energy.
1 The vitamin and mineral premix contained vitamins and trace elements to meet the requirements specified by the National Research Council (1994). The premix provided (units/kg diet): retinol, 12 000 IU; cholecalciferol, 5000 IU; α-tocopherol, 34 mg; menadione, 3 mg; thiamine, 2 mg; riboflavin, 7 mg; pyridoxine, 5 mg; cobalamin, 15 μg; nicotinic acid, 50 mg; pantothenic acid, 15 mg; folic acid, 1 mg; biotin, 200 μg; 80 mg iron as iron sulphate (30%); 10 mg copper as a copper sulphate (25%); 100 mg manganese as manganous oxide (62%); 80 mg zinc as zinc oxide (72%); 1 mg iodine as calcium iodate (52%); 0.2 mg selenium as sodium selenite (4.5%); 0.5 mg molybdenum as sodium molybdate (40%).
2 Analyses were performed in duplicate.
Husbandry and sample collection
In all, 64 male Ross 308-day-old chickens were used in the study. From a day old to the age of 7 days, all birds were reared in a single floor pen and fed a proprietary chicken starter feed that did not contain any coccidiostat or antimicrobial growth promoters, prophylactic or other similar additives. The birds were vaccinated for infectious bronchitis at the hatchery.
On the 1st day of the experiment (7 days of age), all chicks were weighed and allocated to one of the 32 pens, two birds in a pen. Each of the 32 pens had a solid floor with an area of 0.16 m2 that was covered with recycled wood shavings. The recycled litter material was from a previous flock reared for 42 days in the National Institute of Poultry Husbandry, Harper Adams University, which had no obvious health problems, although some sub-clinical necrotic enteritis, coccidiosis or presence of some other pathogens was possible. It has been assumed that the use of recycled litter may impose some additional stress on the birds and may emphasise the effect of the fed mixture of EO. Each diet was offered to birds housed in one of eight pens in a randomised complete block design. The temperature was kept at 29°C at 7 days of age and was gradually reduced to 21°C at the end of the 14-day feeding period (21 days of age). The light regimen was 18 h light and 6 h dark. At 17 days of age, the solid floor of each pen was replaced with a wire mesh and excreta samples were collected for 4 consecutive days from each pen, immediately dried at 60°C and then milled for further analyses. The birds were weighed on a per-pen basis at the beginning, at 7 days old, at the end of the study and at 21 days old; and the average bird feed intake (FI), weight gain (WG) and feed conversion ratio (FCR) were determined. Although the feeding period was 14 days, the birds were in contact with the litter for 10 days only, from 7 to 17 days of age. At the end of the study, at 21 days old, one bird from each pen was stunned/killed, and blood and ileal intestinal samples from one bird per pen were collected for analysing serum amyloid A (SAA), an acute-phase protein, and ileal villus morphometry, respectively. The liver from the same bird was collected and stored at −20°C for further analysis of antioxidant contents.
Chemical analysis
The experimental diets and the excreta were milled (0.75 mm mesh) and analysed further. Dry matter was determined by drying samples in a forced draft oven at 105°C to a constant weight. Crude protein (6.25×N) in samples was determined by dry combustion method (Association of Official Analytical Chemists (AOAC), 2000) using a LECO (FP-528N; Leco Corp., St. Joseph, MI, USA). Oil (as ether extract) was extracted with diethyl ether by the ether extraction method (AOAC, 2000), using a Soxtec system (Foss UK Ltd, Warrington, UK). The gross energy value of the samples was determined in a bomb calorimeter (model 6200; Parr Instrument Co., Moline, IL, USA), and benzoic acid was used as the standard. Minerals in the samples were determined by inductively coupled plasma-emission spectrometry (ICP-OE) (Optima 4300 DV Dual View ICP-OE spectrometer; Perkin Elmer, Beaconsfield, UK) (Tanner et al., Reference Tanner, Baranov and Bandura2002). The N-corrected AME (AMEn) of the diets was calculated as described by Hill and Anderson (Reference Hill and Anderson1958). The coefficients of total tract fat (FR) and mineral retention, dry matter retention (DMR) and nitrogen retention (NR) were determined as the difference between intake and excretion of the nutrient divided by its respective intake.
The concentration of sialic acid (SA) in excreta was determined by the periodate–resorcinol method as described by Jourdian et al. (Reference Jourdian, Dean and Roseman1971).
Concentration of total carotenoids in diets and liver, hepatic coenzyme Q10 and vitamin E (α-, γ- and ϭ-tocopherols) were determined as previously described (Karadas et al., Reference Karadas, Pirgozliev, Rose, Dimitrov, Oduguwa and Bravo2006 and Reference Karadas, Surai, Grammenidis, Sparks and Acamovic2014).
The SAA in blood collected postmortem was determined by a solid phase sandwich ELISA using the Tridelta PhaseTM (Tridelta Development Ltd, Co. Kildare, Ireland) range SAA kit, according to manufacturer recommendations.
Ileal villus morphometry
Approximately 4 cm of the middle part of the ileum, between the Meckel’s diverticulum and the ileoceacl junction, of one of the birds was sampled and stored in 10% neutral buffered formalin saline. The samples then were embedded in paraffin wax, sectioned at ~5 μm and three gut segments were fixed in each slide. Morphometric measurements were determined on 20 intact well-oriented villus–crypt units for each slide (microscope Microtec; TEC Microscopes Ltd, Axbridge, UK; CCD camera Infinity 2, Lumenera Corporation, Ottawa, Canada; Image analysis software, Infinity Analyse – Infinity 2-2 for Windows version 6.5.2, Lumenera Corporation, Ottawa, Canada) as previously described (Abdulla et al., Reference Abdulla, Rose, Mackenzie and Pirgozliev2017).
Oocyst counts
Counts of Eimeria spp. oocysts were determined in excreta samples taken from each pen at 16 days of age, 9 days after the beginning of the experiment. Sampling was carried out by collecting about 20 g samples of excreta, two times per day from each pen. Samples collected from each pen were placed in a separate tub, homogenised thoroughly by a mixer and kept refrigerated for 2 days, until assessed for total oocyst counts. Homogenised samples were ten-fold diluted with tap water to be further diluted with saturated NaCl solution at a ratio of 1 : 10. Oocyst counts were determined using McMaster chambers and presented as the number of oocysts per gram of excreta (Hodgson, Reference Hodgson1970).
Statistical procedures
Statistical analyses were performed using the GenStat statistical software (GenStat 18th edition 3.22 for Windows; International Association for Cryptologic Research, Rothamstead, Hertfordshire, UK). The AMEn content of the experimental diets, broiler growth performance and nutrient digestibility were compared statistically by two-way ANOVA using a 2×2 factorial arrangement of treatments. The main effects were the cereal type (wheat and maize) and the EO supplementation (with and without). In all instances, differences were reported as significant at P<0.05. When a significant F test was detected, means were separated using the Fisher’s protected LSD test. Tendencies towards significance (0.05<P<0.1) were also reported.
Results
The analysed chemical composition of the basal diet is shown in Table 1. The analysed dietary protein content was lower than expected, and the analysed Ca content was lower than expected in the wheat-based diets in particular.
Birds remained healthy throughout the study period and there was no mortality. The weight of the birds fed maize-based diets was 0.690 kg or 12% heavier (P<0.05) than the weight of the birds fed a wheat-based diet, that is, 0.616 kg (data not included in tables). The overall liver weight was 15.6 g and was not influenced by dietary treatments (P>0.05), although when expressed as percent of the BW; the liver of the birds fed maize was 2.32% and of those fed wheat was 2.46% (P<0.05; data not included in tables).
Table 2 shows the data on growth performance, AMEn and nutrient utilisation coefficients. Birds fed maize-based diet had greater daily WG and reduced FCR (P<0.05) compared with wheat-fed birds. However, wheat-based diet tended (P=0.056) to have greater AMEn and had greater FR (P<0.05) compared with maize-based diets. Birds fed the EO-supplemented diets had a lower FCR, using 56 g less feed to produce a kilogram of growth (P<0.05). Daily FI, DMR and NR were not influenced (P>0.05) by dietary treatments.
Table 2 The effect of diet formulation and a commercial bled of essential oil (EO) on feed intake (FI), weight gain (WG), feed conversion ratio (FCR), total tract dry matter retention (DMR), nitrogen retention (NR) and fat retention (FR) coefficients when fed to broiler chickens from 7 to 21 days of ageFootnote 1

AMEn=nitrogen-corrected apparent metabolisable energy.
1 Each value represents the mean of eight replicates.
2 Pooled SEM.
Data on morphological variables of the ileum and excreta SA concentration are presented in Table 3. There were no differences (P>0.05) in villus height, crypt depth and the ratio between them due to EO supplementation or cereal inclusion. Sialic acid concentrations were not affected (P>0.05) by dietary treatments. The overall oocysts count in excreta was relatively low, that is, 5119 eggs/gram fresh excreta, and not affected (P>0.05) by cereal type or EO supplementation.
Table 3 The effect of diet formulation and a commercial bled of essential oils (EO) on morphological variables of the ileum in 21-day-old broiler chickens, sialic acid excretion and excreta Eimeria oocyst countFootnote 1
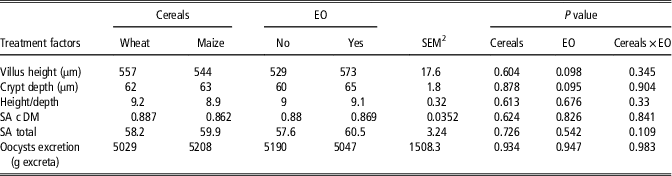
SA c DM=sialic acid concentration in excreta (dry matter); SA total=total sialic acid excreted during the collection period; oocysts excretion=Eimeria oocyst count.
1 Each value represents the mean of eight replicates.
2 Pooled SEM.
Results on dietary mineral retention coefficients are presented in Table 4. Feeding EO improved the coefficients of Ca and Na retention (P<0.05). Feeding wheat-based diets improved the retention coefficients of Ca (P<0.05), P (P<0.05) and Na (P<0.001).
Table 4 The effect of diet formulation and a commercial bled of essential oils (EO) on dietary mineral retention coefficients in broiler chickens (data based on excreta collection from 17 to 21 days age)Footnote 1
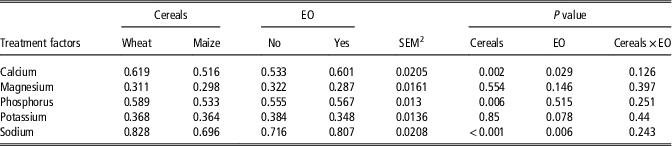
1 Each value represents the mean of eight replicates.
2 Pooled SEM.
Data on hepatic antioxidants and SAA in blood are presented in Table 5. Feeding dietary EO improved (P<0.05) the concentrations of hepatic vitamin E by 53.2%, carotene by 34.3% and coenzyme Q10 by 19.2%, respectively. The hepatic concentration of carotene of the maize-fed birds was 55.6% greater (P<0.05) compared with the birds fed wheat-based diets. No dietary impact (P>0.05) on SAA in blood samples was observed.
Table 5 The effect of diet formulation and a commercial bled of essential oils (EO) on the concentration of hepatic carotene, coenzyme Q10, vitamin E and serum amyloid A (SAA) in blood, determined on 21-day-old broiler chickensFootnote 1
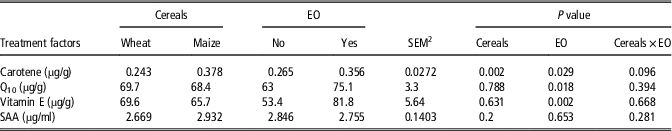
1 Each value represents the mean of eight replicates.
2 Pooled SEM.
Discussion
The analysed dietary protein and Ca contents differed from the calculated values. This was probably due to differences between the composition of the actual ingredients that were used in the present study and the values in the database used by the software for the dietary formulations.
Essential oils, such as carvacrol, cinnamaldehyde and capsicum oleoresin, when supplemented to diets, are known to exert positive effects on the performance and nutrient utilisation in broilers reared in houses with poor hygienic status (Pirgozliev et al., Reference Pirgozliev, Bravo and Rose2014). These effects are likely mediated by a gastrointestinal microbial modification, promoted local protective immunity against Eimeria infection, improved hepatic antioxidative status, dietary energy and nutrient utilisation (Lee et al., Reference Lee, Lillehoj, Jang, Lee, Park, Bravo and Lillehoj2011; Karadas et al., Reference Karadas, Surai, Grammenidis, Sparks and Acamovic2014). The major findings of the current study are the improved FCR and hepatic antioxidant status of the birds fed the EO in both wheat- and maize-based diets. However, Jamroz et al. (Reference Jamroz, Wertelecki, Houszka and Kamel2006) using the same blend of EO reported EO×cereal type interaction on FCR from 0 to 21days age. The EO reduced the FCR in maize-fed birds, although there was no effect in wheat-fed birds, as overall wheat produced lower FCR than maize. This is coupled with reduced crypt depth in maize fed, but not in wheat-fed birds (Jamroz et al., Reference Jamroz, Wertelecki, Houszka and Kamel2006). The intestinal villus morphometry may be linked to the gut health of the birds. Changes in intestinal morphometry such as reduced villous height:crypt depth ratio, involving shorter villi and/or deeper crypts, have been associated with the presence of toxins or higher tissue turnover and poor growth performance (Xu et al., Reference Xu, Hu, Xia, Zhan and Wang2003). The length of the villus in the present study was in the expected range (Abdulla et al., Reference Abdulla, Rose, Mackenzie and Pirgozliev2017), but there were no significant differences in villus morphometry.
Comparing mash v. pelleted diets, Pirgozliev et al. (Reference Pirgozliev, Mirza and Rose2016) found over 20% lower weight in birds fed mash diets, thus partially explaining the relatively low bird weight. The low dietary protein, inclusion of rye and barley, and the rearing conditions (i.e. recycled litter) probably also contributed to the lower than expected bird performance observed in this study. In the present study, the birds fed maize-based diets had an improved daily WG and FCR but had lower AMEn and FR coefficient. The improved growth performance of birds fed maize may partially be explained by the fact that compared with wheat, maize contains less water-soluble NSP – a carbohydrate complex possessing antinutrient activity (Annison et al., Reference Annison, Hughes and Choct1996). Research by Bozkurt et al. (Reference Bozkurt, Küçükyilmaz, Uğur Çatli, Özyildiz, Çinar, Çabuk and Çoven2012) further supports our findings and addresses the impact of NSP on efficacy of the EO. However, Jamroz et al. (Reference Jamroz, Wertelecki, Houszka and Kamel2006) reported no difference in the bird WG when maize- or wheat-based diets are fed. The use of different dietary formulations, rearing conditions and strains of birds may be reasons for the observed differences in response to EO in different studies.
It has been demonstrated that an increase of unsaturated fats in diets improves digestibility of fat and dietary metabolisable energy (Dänicke et al., Reference Dänicke, Simon, Jeroch, Keller, Gläser, Kluge and Bedford1999). Indeed, the wheat-based diet contained more vegetable oil, compared with maize-based diet, thus explaining the response. However, the total fat content in the diet is relatively low (<5%), therefore the difference in FR observed is unlikely to cause major differences in growth performance. The metabolisable energy and nutrient retention response to supplementary EO varies between different reports (Bravo et al., Reference Bravo, Pirgozliev and Rose2011 and Reference Bravo, Utterback and Parsons2014). The inconsistence may be due to different dietary compositions and experimental conditions (Amad et al., Reference Amad, Manner, Wendler, Neumann and Zentek2011). In addition, relatively small differences in dietary metabolisable energy are not always directly consistent with growth performance of birds (Abdulla et al., Reference Abdulla, Rose, Mackenzie and Pirgozliev2017), thus a lack of correlation in the responses to growth and dietary AMEn might be expected.
The number of oocyst output per gram excreta in the reported study is relatively low compared with Eimeria infected birds (Chapman et al., Reference Chapman, Cherry, Danforth, Richards, Shirley and Williams2002) and this suggests that there was no major disease challenge to the birds. This is also indicated by the lack of response of SAA to dietary treatments in this study. The SAA is a major acute-phase protein of the chicken, and is produced predominantly by the liver as a systemic manifestation of the body’s response to inflammation (Eckersall, Reference Eckersall2000). The very low concentration of SAA was in agreement with Chamanza et al. (Reference Chamanza, Toussaint, van Ederen, van Veen, Hulskamp‐Koch and Fabri1999) and showed that no acute inflammation occurs in the chicks. This very low SAA concentration in blood may also be a reason for the lack of impact of the EO blend fed to the birds in this study.
Recent research (Amad et al., Reference Amad, Manner, Wendler, Neumann and Zentek2011) demonstrated that feeding phytogenics improves the digestibility of dietary minerals, including Ca, P and crude ash in poultry. Hosseini et al. (2013) also reported an increase in blood Ca and P concentrations in broilers fed phytogenics. The results on mineral digestibility of the present report are in the expected range (Scholey et al., Reference Scholey, Burton, Morgan, Sanni, Madsen, Dionisio and Brinch-Pedersen2017). The improved digestibility of Ca, K and Na in EO-fed birds coincided with greater hepatic antioxidant content of the birds. It can be hypothesised that dietary EO, in combination with the relatively low dietary levels of these minerals, are likely reasons for the better absorption and digestion reported. The improved hepatic antioxidant content suggests reduced oxidative stress on the birds (Karadas et al., Reference Karadas, Surai, Grammenidis, Sparks and Acamovic2014). This favours gut health and overall animal health and can at least partially explain the observed results. The improved digestibility of Ca, P and Na in wheat-based diets, however, may also be attributed to the relatively low FI of the birds, and the positive impact of the additional dietary fat (Dänicke et al., Reference Dänicke, Simon, Jeroch, Keller, Gläser, Kluge and Bedford1999). Birds fed EO had an increase in Na retention coefficient by 12.7%. Improved Na retention (reduced excretion) was also reported in phytase fed broilers due to phytate hydrolysis and reduced irritation of the gastrointestinal tract (Pirgozliev et al., Reference Pirgozliev, Acamovic and Bedford2009). As the primary cation in extracellular fluids in animals and humans, Na is physiologically important and involved in maintaining the fluid and electrolyte balance in the body (Amad et al., Reference Amad, Manner, Wendler, Neumann and Zentek2011; Hosseini et al., 2013), thus further research clarifying the impact of EO on Na and mineral bioavailability in general is warranted.
In agreement with previous research (Karadas et al., Reference Karadas, Surai, Grammenidis, Sparks and Acamovic2014), feeding the experimental combination of EO improved the hepatic concentration of antioxidants, including carotene, coenzyme Q10 and total vitamin E. It is assumed that the diets are the main determinant of the carotenoid composition in liver tissue (Karadas et al., Reference Karadas, Pirgozliev, Rose, Dimitrov, Oduguwa and Bravo2006). Although there was no difference in feed intake, there was an improved carotenoid concentration in the liver of EO-fed birds, suggesting a better absorption rate and/or less oxidation, thereby preventing carotenoid reserves from depletion. The increased hepatic concentrations of the carotenoids, coupled with increased concentrations of coenzyme Q10 and vitamin E (Karadas et al., Reference Karadas, Pirgozliev, Rose, Dimitrov, Oduguwa and Bravo2006 and Reference Karadas, Surai, Grammenidis, Sparks and Acamovic2014), corresponded to an improved feed efficiency. Carvacrol and cinnamaldehyde (components contained in the additive used in this study) have been previously found to increase the activity of the antioxidant enzymes of the mucosal cells (Dhuley, Reference Dhuley1999), thus reducing the intestinal cell damage and cell turnover and sustaining the integrity of the intestinal mucosal layer. Greater concentrations of antioxidants in body tissues, for example, liver, may also improve health status of the birds and decrease the likelihood of infectious diseases (Salami et al., Reference Salami, Guinguina, Agboola, Omede, Agbonlahor and Tayyab2016). The improved hepatic carotenoid concentration in maize-fed birds may be explained with the greater carotenoid content in maize compared with wheat (Panfili et al., Reference Panfili, Fratianni and Irano2004).
In conclusion, data from this study indicate that a standardised dietary combination of EO, including carvacrol, cinnamaldehyde and capsicum oleoresin, improved the nutritional value of wheat and maize-based diets, when fed to broiler chickens. Although there was no effect of the EO inclusion on the oocysts excretion from the birds, there was no evidence of coccidial challenge in the birds rearing environment, that is, recycled litter. Differences in variables between maize and wheat-based diets are associated with differences in chemical composition between cereals and different amounts of dietary oil. These results demonstrated that the addition of EO in wheat- and maize-based diets provided benefits in terms of feed efficiency, mineral availability and antioxidant status of the birds.
Acknowledgements
The authors thank Richard James and Rose Crocker for their technical support.
Declaration of interest
No potential conflict of interest declared.
Ethics statement
Harper Adams University Research Ethics Committee approved the study.
Software and data repository resources
None of the data were deposited in an official repository.







