Central-line–associated bloodstream infection (CLABSI) is a leading cause of morbidity and mortality among hospitalized patients in intensive care units.Reference Olaechea, Palomar, Alvarez-Lerma, Otal, Insausti and Lopez-Pueyo 1 A recent systematic review found an odds ratio of in-hospital death associated with CLABSI between 1.5 and 2.75.Reference Ziegler, Pellegrini and Safdar 2 In addition, these infections significantly increase treatment costs and length of hospitalization.Reference Blot, Depuydt and Annemans 3 , Reference Higuera, Rangel-Frausto and Rosenthal 4 During the last decade, multifaceted interventions conducted in ICUs in Europe and the United States have resulted in a marked reduction in CLABSI rates. 5 – Reference Van der Kooi, Sax and Pittet 8 Most CLABSI prevention practices are focused on 2 separate bundles of prevention measures: insertion bundles, which minimize the risk of infection related to catheter placement, and maintenance bundles, which reduce the risk of infection during ongoing catheter use. Reference Ling, Apisarnthanarak and Jaggi 9 Insertion bundles include hand hygiene, use of maximal sterile barriers, avoiding the femoral site, and chlorhexidine (CHD) for skin preparation. Maintenance bundles include scrubbing the needleless connector prior to every access, aseptic technique for tubing and dressing changes, maintaining the integrity of the dressing over the insertion site, and line removal as soon as possible. In addition to the basic measures, several supplementary or second-tier measures have been effective, including CHD-impregnated dressings, daily CHD bathing, and alcohol caps.Reference Safdar, O’Horo and Ghufran 10 – Reference Wright, Tropp and Schora 12
A systematic review of 96 studies conducted between 2000 and 2015 revealed a significant association between implementation of insertion and maintenance bundles and reduction in CLABSI incidence in all ICU settings.Reference Ista, van der Hoven and Kornelisse 13 Despite these advances, CLABSI remains a considerable problem in many ICUs. This problem could be due to inadequate knowledge of prevention practices or poor compliance by physicians and nurses. National and multinational surveys have assessed the adoption and consistent use of CLABSI prevention bundles.Reference Krein, Greene and Apisarnthanarak 14 – Reference Furuya, Dick, Herzig, Pogorzelska-Maziarz, Larson and Stone 16 Although most hospitals reported use of maximum barrier precautions, substantial differences both between and within countries have been observed regarding the use of other measures.Reference Krein, Greene and Apisarnthanarak 14 In a survey conducted in medium- and high-income countries, CLABSI prevention guidelines were available in most ICUs.Reference Valencia, Hammami and Agodi 15 Although high compliance was reported regarding some practices, overall compliance to all recommended practices was low, especially in middle-income countries.
CLABSI comprise 35% of acquired bloodstream infections (BSIs) in Israeli ICUs (unpublished data). In 2012, a national CLABSI prevention intervention was initiated, including insertion and maintenance bundles, monthly reporting, and feedback. Following the intervention, a significant decrease was observed in the incidence of both total BSI and CLABSI. Between 2012 and 2016 in medical-surgical ICUs, the pooled mean CLABSI rate fell from 7.2 to 4.0 per 1,000 central-line days, and the pooled mean rate of total BSI fell from 9.4 to 6.7 per 1,000 patient days. Prevalence surveys in Israeli medical-surgical ICUs reported a high degree of compliance with basic guidelines for insertion and maintenance. Nevertheless, CLABSI incidence has remained high in some units. The aims of this study were (1) to characterize the implementation of second-tier prevention measures in Israeli medical-surgical ICUs and (2) to evaluate the association between these prevention measures and CLABSI incidence.
Methods
Online survey
In June 2017, an online survey was sent to physician and nursing staff of infection control units in all acute-care hospitals in Israel with medical-surgical ICUs. Recipients of the survey were asked about CLABSI prevention practices in medical-surgical ICUs in their institutions. Questions focused on 14 second-tier preventive elements within 2 domains: technology and implementation. The 7 technology practices were (1) use of a designated supply cart (2) ultrasound use during insertion (3) site preparation with single-use 2% CHD/70% alcohol (considered a second-tier practice in Israel due to limited availability of this product when national guidelines were published in 2011) (4) utilization of single-use 2% CHD/70% alcohol before accessing the port (5) CHD-impregnated dressings (6) daily CHD bathing and (7) alcohol caps. The last 3 measures were classified as novel technologies. The 7 implementation practices were (1) identifying ward champions (physician and nurse) (2) auditing line insertions and (3) auditing line maintenance by infection control staff (4) e-learning modules (5) annual lectures (6) infection control staff involvement in training and (7) simulation-based training.
Data collection and validation
The CLABSI incidence data were collected as part of routine national surveillance conducted in Israeli acute-care hospitals. We recorded CLABSI rates in medical-surgical ICUs in the first 6 months of 2017. Israel’s national nosocomial infection surveillance program involves mandatory reporting to the Ministry of Health’s National Center for Infection Control (NCIC). Hospitals are required to report monthly all positive blood cultures collected in ICUs, along with the following details: admission and discharge dates, signs and symptoms of infection, diagnostic procedures, as well as dates and presence of central venous lines. Institutional infection control teams have been trained in the use of updated NHSN standardized definitions. 17 An electronic data collection form with built-in algorithms supports the local teams during the surveillance process (eg, via automatic calculation of the infection window period). All reports are reviewed by an infection control preventionist at the NCIC. Whenever a discrepancy is found between an institution’s surveillance assessment and an NHSN definition, the case is clarified with the local team. Survey responses were validated during site visits that the NCIC team conducted in all participating ICUs between September 2017 and February 2018.
Statistical analysis
Hospitals in Israel were divided into 3 categories: tertiary care, medium-sized nontertiary care (≥400 beds), and small nontertiary care (<400 beds). We used ANOVA to compare the number of prevention measures and CLABSI rates (log-transformed because of unequal variances) among the 3 hospital groups. The association between the number of prevention measures and CLABSI rates during the first 6 months of 2017 was assessed using Spearman’s correlation. We used negative binomial regression to calculate the incidence rate ratio (IRR) associated with the overall number of measures and with each prevention measure. Hospitals were categorized into 4 groups based on high or low uptake of technology measures and implementation measures. We used ANOVA to compare the log-transformed CLABSI rate between the 4 groups. The study was approved by the jurisdictional institutional review board.
Results
All 24 acute-care hospitals with medical-surgical ICUs and at least 100 central-line days during the observation period completed the survey. Characteristics of units, CLABSI rates in the first 6 months of 2017, and central-line utilization ratios are shown in Table 1. Large variations in the implementation of second-tier prevention measures were found between facilities (Table 2). Use of a designated supply cart for line insertion was the single measure implemented in all hospitals. The use of at least 1 novel technology was reported by 79% of the facilities. The infection control team provided annual training for physicians and nurses in 46% of the facilities. The number of prevention measures implemented ranged from 3 to 12 of 14 measures. The average number of prevention measures in the tertiary-care hospitals was 9.2, compared with 7.4 in medium-sized hospitals and 4.9 in small hospitals (P = .002).
Table 1. ICU Characteristics and CLABSI Rates in Participating Hospitals, January–June 2017

Note. ICU, intensive care unit; CLABSI, central-line–associated bloodstream infection; SD, standard deviation.
Table 2. Use of Second-Tier CLABSI Prevention Measures and Their Association With CLABSI Rates

Note. CI, confidence interval; CLABSI, central-line–associated bloodstream infection; CHD, chlorhexidine.
During the first 6 months of 2017, the average CLABSI rate was 4.4 per 1,000 central-line days (range, 0–17). The average CLABSI rate in tertiary-care hospitals was low compared with medium-sized and small hospitals (P = .002) (Table 1). The correlation between the number of prevention measures and CLABSI incidence is shown in Fig. 1. A higher number of measures was associated with lower CLABSI rates (Spearman’s ρ = −0.70; P < .001). For each additional measure, the incidence of CLABSI decreased by 19% (IRR, 0.81; 95% CI, 0.73–0.89). The association between individual prevention measures and CLABSI rate is shown in Table 2. Among the 7 technology measures, only CHD bathing approached statistical significance (P = .06). Within the implementation domain, simulation, use of ward champions and audits of line insertions by infection control staff were associated with CLABSI prevention (P < .001, P < .001, and P = .001, respectively); maintenance audits approached statistical significance (P = .06). Figure 2 demonstrates the impact of low (0–3) and high (4–7) uptake of technology and implementation measures. A significant difference was observed between the 4 groups represented (P = .003).
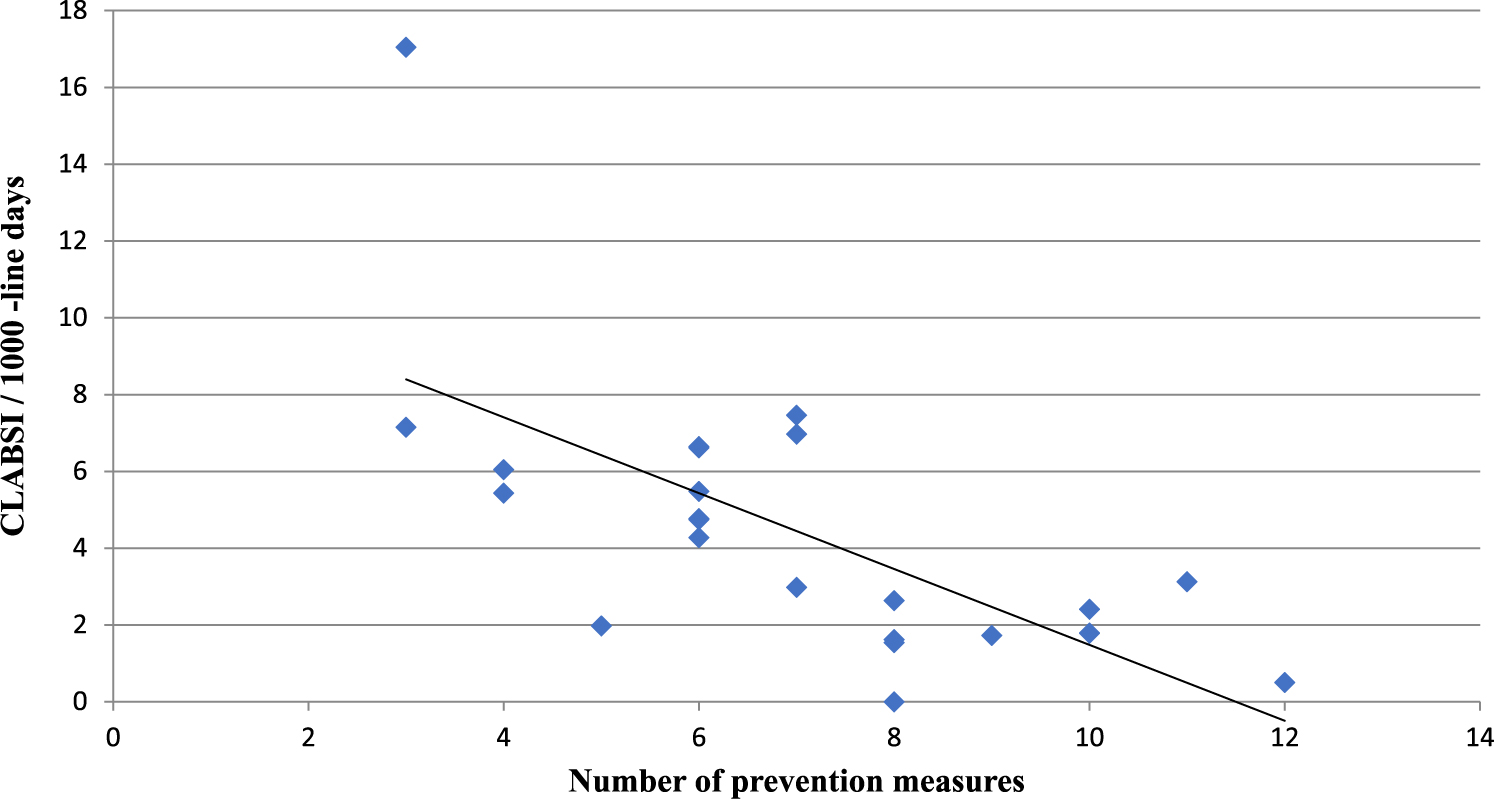
Fig. 1. Correlation between number of prevention measures and central-line–associated bloodstream infection (CLABSI) incidence in medical-surgical ICU in 24 hospitals.
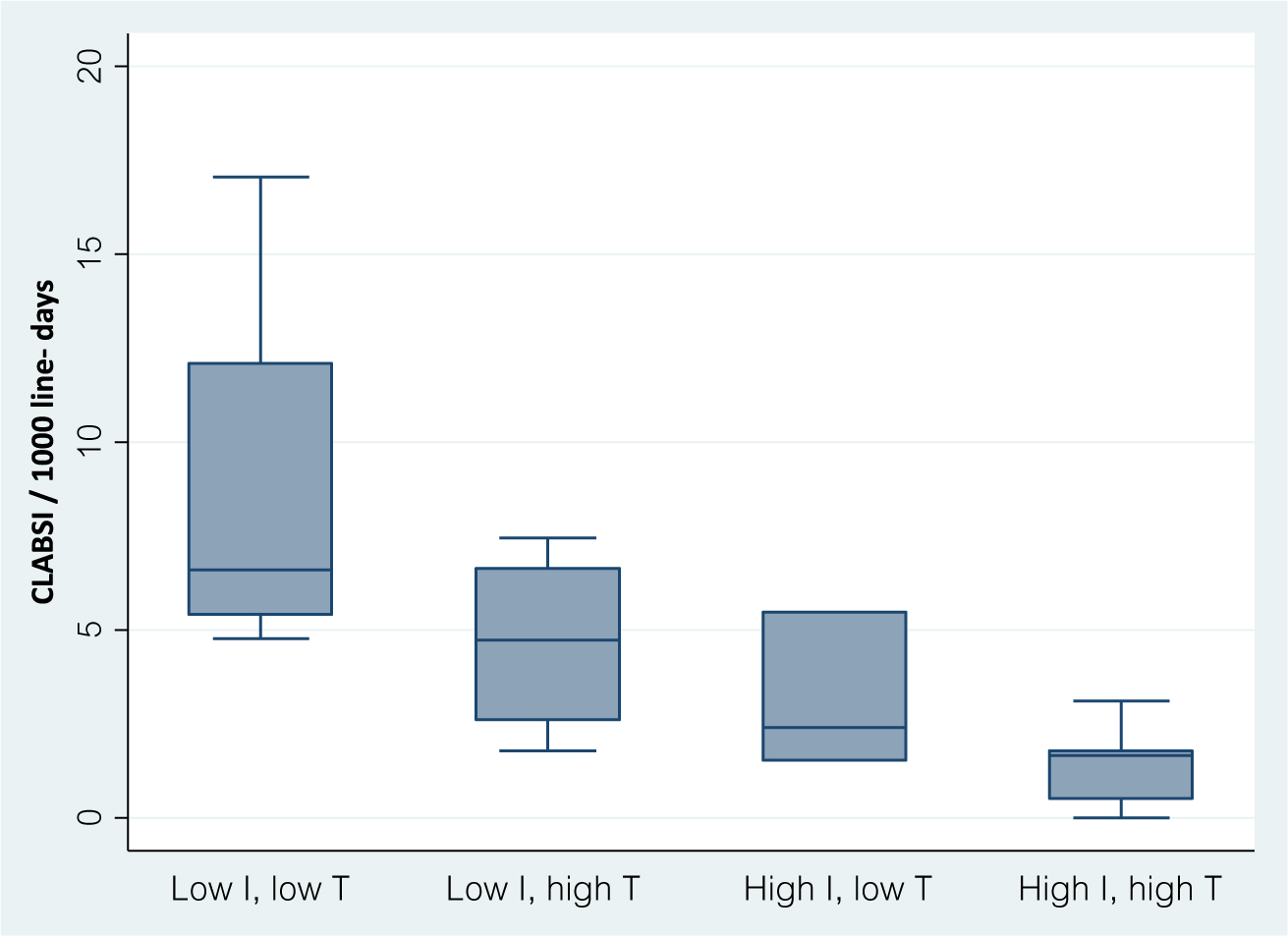
Fig. 2. Box plots of central-line–associated bloodstream infection (CLABSI) rates in hospitals grouped by use of implementation (I) measures and technology (T) measures (low if ≤3).
Discussion
Prevention of device-associated infection represents a complex challenge for the teams involved in device insertion and maintenance and for infection prevention teams. Despite marked strides in disseminating guidelines over the past decade, important gaps remain in the implementation of CLABSI prevention practices. Since the publication of the findings of the Keystone Project more than a decade ago,Reference Pronovost, Needham and Berenholtz 18 the primary bundle of measures outlined in that report (ie, hand hygiene, CHD-alcohol skin preparation, full barrier precautions during line insertion, preference for the subclavian site, and removal of unnecessary lines) has been widely accepted as standard of care for CLABSI prevention. In addition to this bundle, a variety of second-tier measures have been proposed and implemented with the goal of preventing CLABSI, and evidence is accumulating regarding several of these. The current report is among the few national studies assessing the extent of implementation of such measures in ICUs, and their impact in preventing CLABSI.Reference Gonzales, Rocher and Fortin 28 , Reference Piazza, Brozanski and Provost 29 Our results indicate a clear association between number of measures implemented and success in CLABSI prevention.
We observed a large variation between facilities in implementation methods and the use of novel technologies. Most hospitals used at least 1 novel technology. CHD dressings or CHD bathing were adopted by ~70% of the ICUs. In contrast, only 20%–25% conducted routine audits of insertion and maintenance. Notably, tertiary-care hospitals, where CLABSI rates were lowest, reported greater use of prevention measures compared with other hospital categories.
Effective implementation has a key role in the success of CLABSI prevention programs. Among the implementation approaches reported are education, performance assessments, provision of feedback, use of teamwork, and improvements in the overall safety culture.Reference Ista, van der Hoven and Kornelisse 13 Lower CLABSI rates were associated with involvement of unit champions, simulation-based training, and conducting audits of line insertion.
Approximately 60% of the facilities reported that unit champions are involved in their prevention program. The importance of having a champion to lead implementation efforts is well accepted as a quality improvement measure.Reference Shortell, Marsteller and Lin 19 Involvement of team members has played a fundamental role in successful CLABSI prevention interventions by identifying barriers, developing solutions, and promoting best practices.Reference Gurses, Murphy, Martinez, Berenholtz and Pronovost 20 Still, little is understood about the factors that affect the types and numbers of champions required for effective implementation of evidence-based practices. A recent study proposed that >1 champion was needed to bring about behavioral change. Merely appointing champions was ineffective. Successful champions were highly motivated and enthusiastic about the practices they promoted.Reference Damschroder, Banaszak-Holl, Kowalski, Forman, Saint and Krein 21
Staff education is an essential element of CLABSI prevention programs. Most facilities used a variety of educational methods including lectures and/or self-study modules. Nevertheless, among the training measures, only simulations were significantly associated with lower CLABSI rates. Several studies conducted in various professional domains have suggested that hands-on practice through simulation is more effective than traditional education.Reference McGaghie, Issenberg, Cohen, Barsuk and Wayne 22 Simulation programs have been found to increase skill of performance, enhance confidence and reduce the risk of complications at the time of insertion.Reference Peltan, Shiga, Gordon and Currier 23 A number of studies have assessed the impact of simulation-based training on CLABSI rates.Reference Khouli, Jahnes and Shapiro 24 – Reference Zingg, Cartier and Inan 26 The use of simulations of central venous catheter (CVC) insertion significantly reduced CLABSI rates compared with those in control wards.Reference Khouli, Jahnes and Shapiro 24 , Reference Barsuk, Cohen, Feinglass, McGaghie and Wayne 25 A multimodal, hospital-wide intervention including simulator workshops resulted in a significant decrease in CLABSI rates in both ICU and non-ICU wards.Reference Zingg, Cartier and Inan 26 The simulation programs in Israeli hospitals target both physicians and nurses involved in central-line insertion and maintenance practices, regardless of their prior experience. This approach is based on previous studies that have shown that simulation training improved trainee performance irrespective of previous experience.Reference Barsuk, Cohen and Nguyen 27
Another important finding of our survey was that <30% of the facilities conducted routine audits of insertion and maintenance practices. Previous studies from Canada and the United States have shown that units that monitor compliance have the greatest decrease in CLABSI rates.Reference Gonzales, Rocher and Fortin 28 , Reference Piazza, Brozanski and Provost 29 The importance of high compliance was demonstrated in a cross-sectional survey conducted in US ICUs. Compliance of >95% with all elements of the insertion bundle was associated with the greatest reduction in CLABSI rates, but only 20% of ICUs reported excellent compliance with all elements.Reference Ista, van der Hoven and Kornelisse 13 Likewise, in a meta-analysis of the effectiveness of prevention bundles, compliance was measured in fewer than half of the interventions and was found to be suboptimal when it was measured.Reference Ista, van der Hoven and Kornelisse 13 Continuous auditing of compliance may be very resource intensive. Furthermore, it is unclear whether observations may be effectively performed by the ward teams or whether external auditing is necessary; whether observations should include all bundle elements or specific insertion/maintenance measures, and how many observations are needed to influence performance.
We found low adoption of some practices that could reduce both infectious and noninfectious complications. Only 40% of ICUs routinely used ultrasound during central-line insertion. In 2 meta-analyses, the use of real-time ultrasound for CVC insertion, compared with standard landmark placement, led to decreased mechanical complications and reduced cannulation attempts.Reference Wu, Ling, Cao, Wang, Xu and Zeng 30 , Reference Lau and Chamberlain 31 Consequently, practice guidelines recommend the routine use of ultrasound during insertion of CVCs.Reference Rupp, Apfelbaum and Blitt 32 Despite this recommendation, a low prevalence of ultrasound use was found not only in our survey but also in other countries. Reference Valencia, Hammami and Agodi 15
The best antiseptic solution to decontaminate the skin prior to catheter insertion remains a subject of debate. CHD–alcohol provides greater protection against short-term catheter-related infections than does povidone iodine–alcohol.Reference Mimoz, Lucet and Kerforne 33 Consequently, US and UK guidelines recommend the use of 2% CHD in alcohol.Reference O’Grady, Alexander and Burns 34 , Reference Loveday, Wilson and Pratt 35 In contrast, guidelines in France and Israel allow the use of 0.5% CHD concentrations. 36 A number of studies have suggested that 0.5% CHD in alcohol is less effective in eradicating microbes from skin compared with 2% CHD in alcohol.Reference Casey, Itrakjy and Birkett 37 , Reference Adams, Quayum, Worthington, Lambert and Elliott 38 In the present survey, 71% of ICUs used single-use vials containing 2% CHD during line insertion, compared with only 25% for line maintenance. Lower CLABSI rates were observed in ICUs using 2% CHD, yet the difference did not reach statistical significance, possibly due to the small sample size.
This study has a number of limitations. The small number of ICUs in the country could lead to failure to detect the impact of certain measures on infection rates. In addition, the small number of ICUs precluded our ability to evaluate our findings via multivariable analysis. Second, our survey captured limited details on patterns of use of prevention strategies; an element may have been adopted in principle but compliance may be inconsistent. In addition, we did not have data on timing of implementation for each measure. As a result, strategies that were recently and not yet fully implemented may have been erroneously interpreted to be ineffective. Finally, because survey respondents were infection control teams rather than ward clinicians, discrepancies may exist between policy and actual implementation. Alongside its limitations, this study has some notable strengths. First, 100% of the facilities completed the survey. Second, as validation of all CLABSI reports is routinely conducted, differences in CLABSI rates between hospitals are known to be genuine and not due to misclassification.
In conclusion, effective and long-term CLABSI prevention is challenging and requires a multifaceted approach combining evidence-based best practices with implementation strategies. Our study found that the use of multiple second-tier infection control measures was associated with lower CLABSI rates. We observed the importance of incorporating simulation-based training, involvement of unit champions and auditing compliance in CLABSI control programs. An additional finding was that use of some proven preventative measures was relatively low. Awareness of these gaps will inform future national interventions to enhance patient safety.
Author ORCIDs
Debby Ben-David MD, 0000-0003-3267-5478
Financial support
None.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.






