Healthcare-associated infections (HCAIs) affect between 5% and 10% of patients admitted to an acute-care hospital. 1 Strategies to prevent and control HCAIs include adequate decontamination of the healthcare environment. Traditionally, decontamination is conducted using a combination of detergents and disinfectants that are effective for flat surfaces and when applied with care by trained staff. However, liquid disinfectants are not suitable for the surfaces of electrical and electronic apparatus such as monitors and beds with electrical drives.
Newer approaches include fumigation, such as with hydrogen peroxide, are extremely effective because they sterilize virtually everything in a room. However, they are less suitable for routine disinfection because patients and staff need to vacate the area during this process.Reference Abreu, Tavares, Borges, Mergulhao and Simoes 2 Hence, fumigation is not practical in critical-use wards such as intensive care units, and is often reserved for controlling outbreaks.
We have developed a cold-plasma method to complement liquid disinfection methods.Reference Cahill, Claro and O’Connor 3 Patients and staff can remain in clinical areas while it is performed, and it can be applied to the surfaces of electrical and electronic equipment without damaging them. Its large plume (20.3 cm [8 inches] in diameter and 10.2 cm [4 inches] long) covers drip stands and bed frames and ingresses into crevices up to 10.2 cm deep. We report on a preliminary assessment on a busy ward using a prototype suitably designed for that environment.
The cold atmospheric pressure plasma (CAPP) device is comprised of 9 separate plasma jets arranged in a square configuration. The plasma jets are 3 cm apart in the square. Each jet is driven by its own high-voltage, sinusoidal power supply, operating at a peak applied voltage of 2.5 kV at 7 kHz. The plasma operating gas is compressed air that flows into the device at a rate of 21 L per minute.
The effectiveness of the CAPP device to reduce microbial contamination was evaluated using patient tray tables on a busy ward of a tertiary referral acute-care hospital over 8 weeks. The device was placed 1–2 cm from the surface of the tray table, and the plasma was delivered in the form of a cold gas. In this pilot study, the CAPP device was used on patient tray tables for ~5 minutes per tray table (~60 seconds per area). Routine hospital-ward cleaning used 1,000 parts per million (ppm) Teepol (Kent, UK), a multipurpose detergent, and 1,000 ppm Presept (Dorset, UK), a hypochlorite solution applied to all surfaces, including those subjected to the CAPP treatment. For our microbial evaluations, we used Petrifilm (3M, St Paul, MN), an aerobic-count plating system. Over an 8-week period, 480 petrifilms with an area of 20 cm2 (5 per table) were used to sample 12 tray tables (6 control and 6 test samples) on the day before CAPP treatment, immediately after CAPP treatment, and 1 day after CAPP treatment.Reference Claro, O’Reilly, Daniels and Humphreys 4
Statistical data analyses were performed using GraphPad Prism 5.00 software (GraphPad software, La Jolla, CA). The means of 3 independent experiments of the log (CFU/mL) between recovered controls and treatments were compared by 1-way analysis of variance (ANOVA), and Tukey’s multiple comparison tests and Student t tests were also used.
The multiple-jet CAPP device significantly reduced microbial contamination on patient tray tables (P<.05; Figure 1). The levels of microbial contamination detected using Petrifilm were higher on the day before the CAPP decontamination was conducted. These levels were significantly reduced on the tray tables by ~50% with CAPP decontamination. The bacterial levels of contamination increased 1 day after CAPP treatment, as expected.
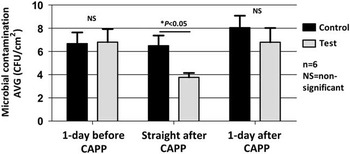
FIGURE 1 Multiple-jet air decontamination of patient tray tables over 8 weeks (n=6; NS, nonsignificant; *P<.05).
Increasing interest in the role of cleaning or hospital hygiene in preventing HCAIs is accompanied by a realization that routine cleaning methods are often ineffective, as are methods for evaluating their effectiveness.Reference Dancer 5 Concurrently, while the emergence of new technologies to augment current cleaning protocols is welcome, their evaluation is also challenging.
Cold atmospheric pressure plasma has the benefit of being nontoxic when used near equipment, patients, and staff, and it has significant antimicrobial properties.Reference O’Connor, Cahill, Daniels, Galvin and Humphreys 6 The antimicrobial effect occurs through the production of reactive atoms as well as positive and negative ions. In an experimental setting, we previously demonstrated the effectiveness of reducing surface bacterial counts on the order of log10 2–3.Reference Cahill, Claro and O’Connor 3 In this pilot study, we extended our evaluation to a surface area commonly found in the clinical arena, the patient tray table. We observed a significant reduction in the average colony-forming units per centimeter, which we believe was not due to other factors. However, this was a pilot study, and a larger evaluation using a variety of surfaces, both flat and irregular, together with an evaluation of the effectiveness of routine cleaning methods are needed.
Recently, some emphasis has been placed on the evaluation of ultraviolet and hydrogen peroxide disinfection systems. In a recent evaluation of pulsed-xenon ultraviolet disinfection, significant log reductions were observed in both spore- and nonspore-forming organisms.Reference Nerandzic, Thota and Sankar 7 A recent evaluation of the impact of vaporized hydrogen peroxide on rates of Clostridium difficile infection using a breakpoint time-series analysis indicated a significant reduction in CDI cases.Reference McCord, Prewitt, Dyakova, Mookerjee and Otter 8 New technologies are likely to augment or even potentially replace current cleaning approaches. However, many of these methods have not been adequately evaluated in appropriate clinical trials, which also consider many variables involved such as patient mix, quality of conventional cleaning, and changes in other infection prevention control measures (eg, hand hygiene). Weber et alReference Weber, Rutala, Anderson, Chen, Sickbert-Bennett and Boyce 9 suggested that a trial comparing ultraviolet light with hydrogen peroxide should be undertaken. However, both methods require the clinical area to be vacated by patients and staff. In a busy clinical setting, a system that would obviate the need for this evacuation should also be explored. Moreover, CAPP, which does not require evacuation, should be further evaluated in a clinical trial to determine how much it enhances hygiene in addition to routine cleaning and whether it reduces nosocomial infections.
ACKNOWLEDGMENTS
Financial support: This research was supported by a grant from the Health Research Board Ireland and Science Foundation Ireland (grant no. TRA/2010/4/10).
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.



