The incidence of Clostridium difficile infection (CDI) has increased markedly in the past 20 years,Reference DePestel and Aronoff 1 associated with the emergence of more virulent strains of the pathogen Clostridium difficile infection is now the most common cause of healthcare-associated infection in the United StatesReference Miller, Chen and Sexton 2 and accounts for considerable morbidity and mortality, especially in patients who experience recurrent disease. The major risk factors for CDI are hospitalization, exposure to broad-spectrum antimicrobials, and advanced age.Reference DePestel and Aronoff 1
Fecal microbiota transplantation (FMT) is increasingly available for the treatment of recurrent CDI. The mechanism of FMT is believed to be through colonization resistance—the restoration of healthy colonic microflora that occupies the ecological niche of the distal gut and prevents C. difficile overgrowth. Since it was first used to treat patients with pseudomembranous colitis in 1958,Reference Eiseman, Silen and Bascom 3 FMT has been shown to be highly efficacious for recurrent CDI, resulting in resolution of symptoms in a higher proportion of patients than antibiotics alone.Reference van Nood, Vrieze and Nieuwdorp 4
The utility of a specialized therapeutic approach is influenced not only by the need for the approach but also the accessibility to the service. Although FMT is highly efficacious and becoming progressively more available, the patients with CDI who are more likely receive FMT remains unknown. We hypothesize that certain clinical features and patient characteristics associated with the first episode of CDI set the stage for the eventual use of FMT. In this single-center study, we set out to investigate the factors associated with the first episode of CDI that predict increased risk for the use of FMT in a cohort of patients with recurrent CDI. We also investigated patient characteristics that may affect access to the procedure.
PATIENTS AND METHODS
Data Collection
A retrospective study was performed on a cohort of adult patients who received FMT for recurrent CDI (group A). They were compared to a second population of adult patients who had suffered at least 3 episodes of CDI over the same period but did not receive FMT. We chose to only include those with 3 or more episodes of CDI for analysis because most of the patients who underwent FMT had experienced at least 3 episodes of CDI. Patients assigned to group A were identified from the database of patients who were referred for and underwent FMT (Group B). Patients assigned to group B were identified using the ICD code for “intestinal infection due to Clostridium difficile,” (ICD 008.45) from a list of all hospital admission diagnosis that included CDI. Data were obtained in both groups from the electronic medical records of inpatients presented between 2006 and 2016 to an academic teaching medical center. Fecal microbiota transplantation was carried out in all cases by colonoscopy. Baseline characteristics of patients in both cohorts were recorded: age; racial or ethnic background; comorbid medical conditions such as heart disease, chronic lung disease, diabetes mellitus, chronic renal disease, gastroesophageal reflux disease, inflammatory bowel disease, and malignancy; and use of immunosuppressive therapy and other medication use. Antibiotic exposure before each episode of CDI was assessed, as well as the choice and duration of treatment of CDI in each case. The Lifespan Institutional Review Board approved this study.
Diagnosis of CDI
In all cases, the diagnosis of CDI was only made after excluding other diarrheal pathologies, and confirmed by the detection of either the Clostridium difficile toxin A or B for cases presented before 2009, or the toxigenic Clostridium difficile by polymerase chain reaction for cases presented in 2009 or then after, when the molecular method was introduced into the microbiology laboratory for routine clinical use.
Statistical Analysis
Association of potential risk factors in the use of FMT was evaluated using the χ2 test. Logistic regression was used to adjust for potential covariates. Predictors that attained P<.05 on univariate analysis were included for multivariable Cox regression. Differences in age and intervals between first and second episode of CDI were calculated as means and compared using Student t tests. A 2-sided P value<.05 was considered statistically significant, and a P value >.05 but <.10 was considered trending toward statistical significance.
RESULTS
Patient Characteristics
A total of 275 patients were included in the analysis. Patients were divided into 2 groups: group A consisted of 200 consecutive patients who received FMT for recurrent CDI and group B consisted of the first 75 consecutive patients who had recurrent CDI during the same periods of time but did not receive FMT. We chose to include 75 patients in group B so the ratio of patients who underwent FMT for recurrent CDI to patients who had at least 3 episodes of CDI but did not receive FMT was approximately 3:1. The study cohort consisted of 192 females and 83 males. Group A consisted of 81.5% females and group B consisted of 65% females. The median ages were 66.5 years (range, 19–97 years) in group A and 64 years (range, 22–97 years) in group B. We detected no statistical difference in the sex and age distribution between the 2 groups.
Premorbid Factors Associated With Increased Risks for FMT
We first determined the factors associated with the first episode of CDI that predisposed patients to FMT. The following risk factors were examined: systemic use of immunosuppressive therapy (IST) and corticosteroids, use of metronidazole and intravenous vancomycin within 2 months of developing the first episode of CDI, and history of chronic leukemias, lymphomas, inflammatory bowel disease (IBD), colon cancer, and HIV (Table 1). Univariate analysis identified the use of IST (P=.04; OR, 3.4; 95% CI, 1–11.6), history of IBD (P=.002; OR, 5.8; 95% CI, 1.7–19.3), and the prior use of metronidazole (P=.02; OR=8.5; 95% CI, 1–66) to be significantly associated with increased risks for subsequent need for FMT. Interestingly, the use of intravenous vancomycin within 2 months of the development of the first episode of CDI predicted a reduced risk for the subsequent need for FMT (P=.000003; OR, 0.08; 95% CI, 0.03–0.24). We did not find prior use of the following antibiotics to be predictive of subsequent FMT use: cefepime, piperacillin/tazobactam, ciprofloxacin, aztreonam, ceftazidime, bactrim, clindamycin, azithromycin, levofloxacin, and ceftriaxone. Multivariate analysis found that prior use of intravenous vancomycin was the only parameter to be positively predictive of FMT use, and the prior use of metronidazole trended toward significance (Table 2).
TABLE 1 Univariate Analysis for Risk Factors for Fecal Microbiota Transplantation in Patients With Recurrent/Refractory Clostridium difficile Infection

NOTE. NS, not significant; IBD, inflammatory bowel disease; HIV, HUMAN immunodeficiency virus; IST, immunosuppressive therapy.
TABLE 2 Multivariate Analysis for Risk Factors for Fecal Microbiota Transplantation in Patients With Recurrent/Refractory Clostridium difficile Infection (CDI)
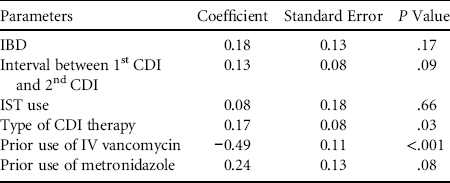
NOTE. IBD, inflammatory bowel disease; IV, intravenous; IST, immunosuppressive therapy.
Therapy Type for First CDI and Subsequent FMT Use
We next sought to determine whether the type of therapy given to the patients for the first episode of CDI predicted subsequent FMT use. Compared to metronidazole (oral or intravenous), patients who required oral vancomycin were nearly twice as likely to need FMT (P=.02; OR, 1.9; 95% CI, 1.1–3.4) if they developed recurrent CDI in both univariate (Table 1) and multivariate analyses (Table 2).
Intervals Between First and Second CDI and Subsequent FMT Use
We postulated that patients who developed a second CDI promptly after recovering from the first episode were also more likely to end up needing FMT. Therefore, we assessed the time intervals between CDI episodes in the 2 groups of patients. The median time between first and second CDI was significantly shorter (P=.00004) in group A than in group B (median, 1 month; range, 0.5–24 vs median, 2 months, range, 0.5–57). Multivariate analysis found that the intervals between first and second CDI trended toward significance (Table 2).
Clinical Characteristics Affecting Access to FMT
Finally, we sought to determine whether certain clinical characteristics affect access to FMT. We found that black patients with recurrent CDI were less likely to receive FMT than white patients (Table 3). FMT was also less likely to be accessed by patients with medical comorbidities, including history of myocardial infarction, congestive heart failure, dementia, chronic renal disease, chronic obstructive pulmonary disease, diabetes mellitus, chronic hepatitis, connective tissue disease, and cancer.
TABLE 3 Clinical Characteristics Affecting Accessibility to Fecal Microbiota Transplantation
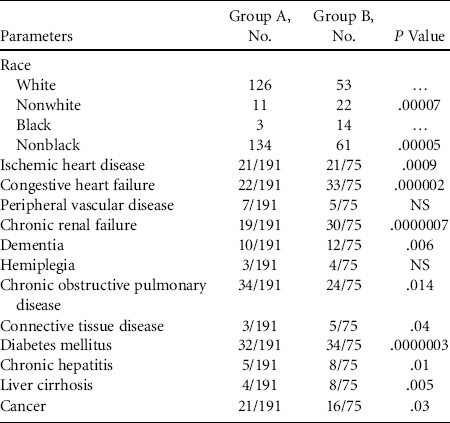
NOTE. NS, not significant.
DISCUSSION
In this retrospective study, we sought to determine the risk factors predictive of the subsequent need for FMT and to examine the clinical characteristics that may hamper access to FMT in patients with recurrent CDI. Identification of these risk factors and clinical characteristics will provide insights into not only approaches to negate the risk factors but also the design of novel strategies to increase accessibility to this therapeutic approach.
Interestingly, exposure to intravenous vancomycin in the 2 months prior to the first CDI significantly reduced the likelihood of eventual FMT. This association most likely originates from the bactericidal effects of the small amount of vancomycin excreted via the hepatobiliary route in patients receiving intravenous vancomycin. Low levels of vancomycin have been detected in the stools of most patients who received intravenous vancomycin.Reference Currie and Lemos-Filho 5 Although the concentrations are normally subtherapeutic against most gram-positive organisms, these low concentrations may be sufficiently higher than the minimum inhibitory concentrations for certain more virulent strains of C. difficile, possibly allowing for easier clearance of the infections with conventional therapies when CDI occurred later. This finding contrasts with our previous work showing the predisposition to acquisition of vancomycin-resistant enterococcus in patients with acute myeloid leukemia treated with intravenous vancomycin.Reference Heisel, Sutton and Mascara 6 In that study, we hypothesized that the intestinal vancomycin level from hepatobiliary excretion was not high enough to mediate bactericidal effect on the enterococci but enough to induce the development of resistance.
We identified the use of either intravenous or oral metronidazole in the 2 months before first CDI to be associated with an increased risk for FMT use. This association may seem counterintuitive because metronidazole has long been used as first-line treatment for CDI. However, recent clinical evidence suggests that patients treated with metronidazole for initial CDI have lower cure and higher mortality rates than those given vancomycin.Reference Stevens, Nelson and Schwab-Daugherty 7 The use of metronidazole in the 2 months before initial CDI may select for the more virulent strains of the bacterium, predisposing patients to recurrent and more severe infections that ultimately required FMT.
The use of oral vancomycin to treat the first CDI was predictive in our study of higher likelihood of requiring FMT. Because oral vancomycin is used primarily for severe CDI, this association is not surprising. It suggests that if the first CDI was severe, medical treatment of CDI was less likely to succeed and the CDI was more likely to recur. The same reasoning applies to patients whose CDI relapsed promptly after treatment for the first episode, reflecting the resistant nature of the disease to medical therapy. These associations raise the question of whether these groups of patients should be treated with a more prolonged course of oral vancomycin or with newer agents, such as fidaxomicin.
Patients on immunosuppressive therapy (IST) have alterations in intestinal microbiota, increasing their risk of developing CDI.Reference Kim, Wang and Sun 8 , Reference Tourret, Willing and Dion 9 Since IST use is long term in most patients, the intestinal dysbiosis often persists after the initial successful treatment of CDI. These patients are more likely to have disease recurrence, rendering them at higher risks for FMT. Patients with IBD also experience chronic intestinal dysbiosis.Reference Maloy and Powrie 10 Therefore, when the patients develop CDI, restoration of normal intestinal microbiome does not occur even with adequate medical therapy and the continued state of intestinal dysbiosis predisposes these patients to recurrent CDI. Consequently, we observed an association between IBD and need for FMT in patients with recurrent CDI.
Having determined the risk factors for FMT use, we then determined the clinical characteristics that may hamper the access of these patients to FMT. Interestingly, although black patients are more likely to develop severe CDI and die from the disease,Reference Argamany, Delgado and Reveles 11 and may be more susceptible to recurrent CDI,Reference Freedberg, Salmasian and Friedman 12 they were significantly under-represented in patients who received FMT for recurrent CDI in our study. We suspect that this discrepancy was due to differences in the access of FMT between blacks and whites, which may be caused by disparities such as health insurance coverage, clinical access, and adequacy of long-term follow-up. Our findings are in keeping with recent studies showing health disparity and consequently use of medical services for CDI among different races.Reference Argamany, Delgado and Reveles 11 , Reference Yang, Rider and Baehr 13 Alternatively, due perhaps to some cultural belief, black patients may find FMT less acceptable, especially when performed by colonoscopy. Therefore, they did not want to be referred for FMT. The less acceptability may be related to the feelings of violation, embarrassment or shame, and fear of procedure-related pain. Such health disparity has previously been reported on the much lower rate of utility of colonoscopy and flexible sigmoidoscopy to screen for colon cancer among black patients.Reference Carethers 14
Our study also found that patients with ischemic heart disease, chronic renal failure, diabetes mellitus, liver cirrhosis, dementia, chronic hepatitis, connective tissue diseases, cancer, and chronic obstructive pulmonary disease were less likely to receive FMT. Although FMT is not a major procedure, our findings suggest that there is probably a bias against referring patients with comorbidities for FMT. Appropriate education provided to referring physicians may increase the access of these patients to FMT.
In this study, we identified the risk factors for use of FMT in patients with recurrent CDI, based on the clinical features of the first episode of CDI. Despite the shortcomings associated with a retrospective study, our findings should serve as a platform to alter the management of patients with CDI to negate their risk factors for recurrent infection. We also found that blacks were less likely to receive FMT for recurrent CDI than whites, most likely due to discrepancies in accessing health care. Such a finding highlights the need to overcome the healthcare disparity around this effective treatment for recurrent CDI.
ACKNOWLEDGMENTS
Financial support: No financial support was provided relevant to this article.
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.




