Clostridium difficile infection (CDI) is the leading infectious cause of antibiotic-associated diarrhea. In 2011, there were an estimated 453,000 cases of CDI in the United States, and approximately 6.5% of these patients died. A substantial number of people experience recurrent CDI (RCDI).Reference Bassetti, Villa, Pecori, Arzese and Wilcox 1 , Reference Shields, Araujo-Castillo, Theethira, Alonso and Kelly 2 The annual rate of RCDI is between 75,000 to 175,000 cases.Reference Shields, Araujo-Castillo, Theethira, Alonso and Kelly 2 Sheitoyan et alReference Sheitoyan-Pésant, Abou, Pepin, Marcil-Héguy, Nault and Valliquette 3 documented a 24.9% risk for recurrence after the first episode of CDI and a 38.3% risk for subsequent recurrences.
Despite detailed information about the increasing prevalence and costs associated with CDI and RCDI,Reference Shah, Aitken and Barragan 4 , Reference Desai, Gupta and Dubberke 5 very little is known about the impact that infection may have on patients’ lives. Persons with CDI most commonly experience symptoms that include diarrhea, abdominal pain, fatigue, headache, fever, light-headedness, nausea, and weight loss.Reference Cohen, Gerding and Johnson 6 These symptoms may last for several weeks and may have lasting effects on the behaviors and quality of life of these patients.
Two studies examined the impact of CDI on patients’ lives. Madeo and BoyackReference Madeo and Boyack 7 interviewed 15 patients with CDI in an acute care hospital, and 4 key themes emerged: (1) physical suffering, (2) lack of control over bowel function, (3) lack of understanding of their illness, and (4) loss of privacy and dignity. Diarrhea was a significant concern, but patients were uncomfortable and embarrassed to discuss it. Loneliness and depression were also common. To understand patients’ experiences from initial symptoms through hospitalization and return to home, Guillemin et alReference Guillemin, Marrel and Lambert 8 conducted interviews with 24 patients who had CDI. Although the results varied among patients, continuous and uncontrollable diarrhea from CDI had the most impact on patients’ lives. Hospitalization increased the emotional distress of these patients. Following discharge and symptom improvement, many patients continued to worry about recurrence and were more careful about what they ate and their hygiene.
The current study focused on individuals who had RCDI to better understand how patient behaviors were impacted by their illness.
METHODS
Study Design and Setting
We conducted a telephone survey of patients with prior RCDI from 7 Chicago area hospitals that are part of the Patient Centered Outcomes Research Initiative (PCORI) Clinical Research Data Network (CDRN) called CAPriCORN (Chicago Area Patient Reported Outcomes Research Network).Reference Solomonides, Goel and Hynes 9
Study Population and Recruitment
Recurrent C. difficile infection cases were identified utilizing a computer algorithm applied to the electronic medical record of each participating facility. We defined RCDI as a second episode of Clostridium difficile within 15–56 days of an initial CDI episode, looking back from October 31, 2015, to January 1, 2013. We used a combination of lab tests (polymerase chain reaction and enzyme immunoassay) and/or diagnostic codes (International Classification of Disease, Ninth Revision [ICD-9] code 008.45) to identify cases (see online Appendix A for the full algorithm). RCDI was validated using manual medical record review by infectious disease clinicians and project staff.Reference Bauer, Kujiper and Van Dissel 10 Staff conferred with clinicians if they were unsure of CDI diagnosis. Only cases with positive lab results for CDI were considered valid cases.
Consent to contact eligible patients was obtained from healthcare providers. Patients were contacted by mail with an invitation to participate in a telephone interview about having had RCDI or diarrhea-related illnesses. Individuals could opt out by returning a postcard indicating that they did not want to be contacted or by calling the research assistant. Telephone interviews were conducted only after verbal consent was obtained.
Survey
The first set of 5 questions regarding current general health status (ie, physical, mental, quality-of-life) were obtained from PROMIS health measures system (http://www.healthmeasures.net/explore-measurement-systems/promis). Additional PROMIS items regarding depression, fatigue, sleep, daily activities, and social isolation were also posed. CDI-specific questions included hospitalization for CDI in the past 3 years, the number of days they had been unable to participate in normal activities due to CDI, and whether they recalled taking an antibiotic or medications for heartburn or gastric reflux prior to becoming ill. Respondents estimated the number of days they were unable to participate in normal activities due to CDI in the past 3 years, and listed the symptoms they experienced when ill. Respondents were also asked to rate the severity of symptoms from their most recent CDI episode on a Likert scale: 1 (mild) to 5 (severe) (or 0 if they did not have the symptom). Symptoms included diarrhea, abdominal pain, nausea, fever, headache, physical exhaustion/weakness, and unexplained weight loss.
The survey included 13 items related to emotional distress identified from the Guillermin study,Reference Guillemin, Marrel and Lambert 8 including fear of getting sick again, fear of getting others sick, shame/embarrassment, and anger. For each item, we created a question for which the respondent indicated how worried or distressed they felt when they had their most recent CDI episode: 0 (not worried/distressed) to 5 (very worried or distressed). The next set of items was developed based on the literatureReference Madeo and Boyack 7 , Reference Guillemin, Marrel and Lambert 8 and experiences of patients and providers related to attitudes toward infection prevention. Respondents indicated how each statement reflected their behavior since their illness on a 5-point Likert scale: 1 (not at all) to 5 (very much). The activities included increased: (1) cleaning around my home, (2) use of bleach, (3) washing hands, (4) use of antibacterial gels and/or wipes, and (5) use of soap and water. Other activities included (1) changed what I ate, (2) ate out less, (3) avoided certain medications, (4) avoided shared public areas, and (5) used probiotics.
Demographic questions included respondent age, gender, race, education, household size, marital status, country of birth, current employment status, total annual household income, and health insurance coverage. The survey is available in online Appendix B. The study was approved by the Chicago Area Central Institutional Review Board formed as a central review body for CAPriCORN-related projects.
Statistical Methods
Ratings of CDI symptoms and distress feelings were tabulated using symptom severity and level of worry, respectively. The extent of change in prevention behaviors was also tabulated.
Generalized linear mixed-effects models were used to estimate the odds of hospitalization for CDI as a function of respondent characteristics, including age, gender, race, education, marital status, primary language, country of birth, employment status, income, household size, and insurance status. A similar model was used to estimate the odds of hospitalization for those with high versus moderate-to-low diarrhea severity. In these models, a logit link was used to estimate the odds ratio (OR), and random intercepts were allowed for each hospital to account for the clustering of patients within their treatment facility.
A generalized linear mixed-effects model also was used to estimate the odds of having more days of inactivity as a function of high versus moderate-to-low diarrhea severity. In addition, to allow random intercepts for each hospital, this model treated the outcome (days of inactivity) as an ordinal variable comprising 4 levels: 0 days, 1–7 days, 8–30 days, and >30 days. A cumulative logit link was used to estimate the odds ratio. Using a similar approach, we also investigated whether respondents with any days of inactivity had higher levels of agreement with the statements about prevention behaviors when compared to those with zero days of inactivity and whether those with high diarrhea severity had higher levels of agreement with such statements when compared to those with moderate-to-low diarrhea severity. For these ordinal models, we assessed the proportional odds assumption.Reference Agresti 11 All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
The RCDI algorithm identified 1,132 possible RCDI cases from 7 hospitals. Several sites excluded deceased cases prior to validating them (15.8%), leaving a total of 953 cases that underwent medical record validation. Of the cases that were reviewed, 39% (372 of 953) were validated as meeting the definition of RCDI. The most common reasons for exclusion were (1) no evidence of 2 separate CDI encounters (28.5%), (2) CDI not confirmed by lab testing (10.9%), (3) did not have 2 CDI encounters within the 15–56-day period (8.4%) or (4) had other reasons for not being validated (11.4%; eg, missing records). Interviews were conducted with 119 patients (32.0%; includes 5 incomplete interviews). The most common reasons for nonresponse included patients who declined to participate (24.6%) and patients we were unable to reach (18.7%). Seven patients (1.9%) did not recall having had CDI and were excused from completing the survey.
The mean age of respondents was 57.4 years (standard deviation, 16.8; median, 60.5 years; range, 18–90 years), just over 50% were female, and 50% said that they had been hospitalized with CDI or diarrhea-related illness in the past 3 years (Table 1). These patient characteristics were similar between those who were and were not hospitalized (all P>.05).
TABLE 1 Patient Characteristics by Whether They Reported Being Hospitalized for Clostridium difficile Infection (CDI)
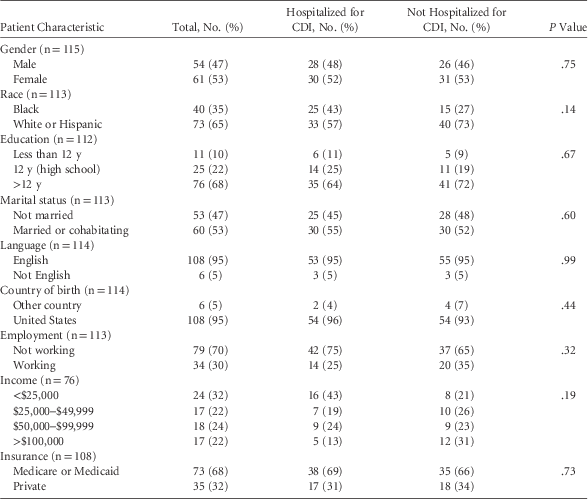
The median number of days patients were unable to participate in normal activities was 12 (range, 0–365 days). Moreover, 58.5% rated their diarrhea as severe, 30.7% reported severe exhaustion, and 29.1% reported having severe abdominal pain. Other symptoms were less severe and/or common.
To examine the relationship between hospitalization, self-reported diarrhea severity and days unable to participate in regular daily activities because of their illness, we combined ratings of diarrhea severity into 2 groups: severe (rating of 4 or 5, 70.5%) and moderate/low (rating of 3 or less, 29.5%). Respondents with severe ratings of diarrhea had 3.08 times higher odds (95% confidence interval [CI], 1.32–7.19) of more days of inactivity when compared to respondents with moderate/low diarrhea severity (P=.01). Similarly, we determined that those with severe diarrhea had higher odds of being hospitalized compared to those with moderate-to-low severity (OR, 3.30; 95% CI, 1.36–8.02; P=.01).
Respondents reported that they were most worried about getting sick again (55.6%) and about being contagious (37.3%) (Table 2). Fear of dying and worries about intimacy were a serious concern for only 17% and 17.8% of respondents, respectively.
TABLE 2 Worries Related to Recurrent Clostridium difficile InfectionFootnote a
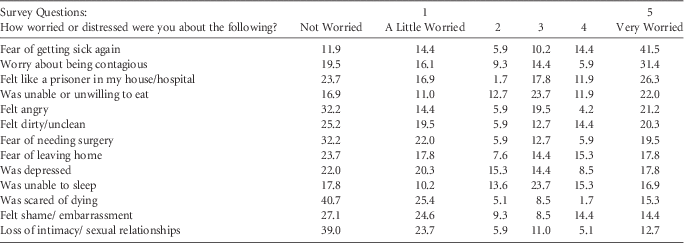
a N=118; all values shown indicate %.
Participant responses related to changing behaviors to prevent infection tended to fall into extremes; either the behavior did not change or it changed a great deal (Table 3). Almost half of the respondents said they wash their hands much more frequently (46.7%), and 44.9% said they had greatly increased their use of soap and water. Other cleaning-related behaviors (ie, use of bleach, housecleaning, use of antibacterial wipes and/or gels) were identified as changing a great deal for at least 33% of respondents. Respondents also reported changing their health-related behaviors; between 22% and 32% indicated that they had greatly changed what they ate, that they ate out less, that they avoided certain medications, that they avoided public areas, and/or that they increased their use of probiotics due to CDI.
Although not statistically significant, there were a few trends in prevention behaviors by disease severity. Respondents who were hospitalized were nominally more likely to have changed their eating habits when compared to those not hospitalized (OR, 1.95; 95% CI, 0.99–3.87; P=.055). Those with severe diarrhea showed a trend toward increased use of soap and water when compared to those with moderate-to-low diarrhea severity (OR, 2.10; 95% CI, 0.97–4.51; P=.058). Finally, compared to respondents with zero days of inactivity, those with 1 or more days of inactivity had significantly higher odds of increased use of bleach (OR, 3.43; 95% CI, 1.30–9.05; P=.01).
DISCUSSION
Persons who contract CDI often experience symptoms that are painful, debilitating, and/or embarrassing. Our survey of patients with prior RCDI confirmed these symptoms. Diarrhea, exhaustion, and abdominal pain were most likely to be rated as severe, whereas the presence and severity of other symptoms such as fever and headache were more variable. Approximately 50% of the sample indicated that they were hospitalized with CDI, and the number of days in which respondents estimated that they were unable to perform routine daily activities because of CDI varied considerably.
Our data also support the findings that having RCDI caused most patients to worry about being contagious and getting sick again. Some were angry and/or depressed, or had difficulty sleeping. These worries and concerns negatively affect quality of life. Garey et alReference Garey, Sethi, Yadav and DuPont 13 developed and validated a CDI health-related quality-of-life questionnaire that includes all the worries and concerns that we identified in our survey. The final version of the measure, the Cdiff32, could discriminate quality-of-life score differences in patients with primary versus RCDI and assess quality of life with increasing time since the last CDI episode. The Cdiff32 covers 3 domains: (1) physical, mental, and social relationships; (2) the subdomain of general and specific physical complaints; and (3) the subdomain of current and future anxiety. While the Cdiff32 was developed primarily with input from providers, our study supports these constructs from the perspective of patients who have had RCDI.
Because CDI can have a substantial effect on patients when they are ill, we were interested in whether it affected their subsequent behaviors. We examined whether patients changed preventive behaviors due to having RCDI. We found that RCDI did change behavior for many respondents. More than 50% said that they greatly increased their handwashing and use of soap and water for bathing, and approximately 50% increased their house cleaning, use of bleach, and use of antibacterial wipes and gels compared to their behavior prior to being ill. While less frequent, at least 33% of respondents said they changed what they ate (eg, avoiding or adding certain foods), avoided taking certain medications such as antibiotics and medications for heartburn, and increased the use of probiotic supplements. A similar proportion said that they avoided public places such as public bathrooms and buffets at restaurants, and that they ate out less since their illness. Interestingly, we found little correlation between diarrhea severity, hospitalization, or days of inactivity and these subsequent behaviors. Because these data were collected in a structured telephone survey, we were unable to verify these responses to determine how much respondents had truly changed their behaviors.
Increased hand washing and use of soap and water for cleaning are evidence-based strategies that have been demonstrated to significantly reduce the likelihood of acquiring and transmitting infections. 14 Short of becoming a compulsion, these behaviors should be encouraged. Other behaviors that patients endorsed have limited or no strong evidence to support them. At this point, there is very little evidence to suggest that changes in diet reduce the risk of contracting CDI. While there is an increased risk of irritable bowel syndrome (IBS) following infectious gastroenteritis,Reference Rodriguez and Ruigomez 15 published data on how diet may affect CDI or IBS development are scarce. One study with mice suggests that a low protein diet may be protective against CDI,Reference Moore, Pinheiro and Zaenker 16 but further research is needed with humans before any recommendation can be made. It is possible that patients with transient symptoms changed their eating habits (eg, ate yogurt) to help with their symptoms and it became part of their ongoing diet. CDI is restricted to the colon, and it is unlikely to affect absorption or any other small-bowel function as is seen following infection with small bowel pathogens such as Giardia spp.Reference Scott, Logan, Klammer, Teoh and Buret 17 Respondents who said they eat out less and/or avoid shared and public places may be adversely impacting their quality of life without changing their risk of future illness. Providers should discuss these behaviors with their patients and should assure them that with appropriate preventive behaviors, such as handwashing, they can still participate in these other activities.
In our cohort, nearly 50% of our patients with RCDI were not concerned about avoiding certain medications. The literature has shown that proton pump inhibitors (PPIs) increase the risk of CDI, both incident cases and recurrences.Reference McDonald, Milligan, Frenette and Lee 18 Further, antibiotics also increase the risk of CDI, and when they are combined with a PPI, the risk is even greater.Reference Freedberg, Lamousé-Smith, Lightdale, Jin, Yang and Abrams 19 , Reference Kwok, Arthur, Anibueze, Singh, Cavallazzi and Loke 20 Physicians should consider the indications for antibiotic use and PPIs in patients with RCDI. In addition, they should encourage their patients to ask about future antibiotic use, and they should empower patients to have discussions about the risks of RCDI and benefits of antibiotic treatment. In addition, healthcare providers should remind patients to consult with them before stopping a medication (eg, a PPI) because the risks may outweigh the benefits.
Finally, the use of probiotics to prevent CDI has received a great deal of attention in the media. More than 33% of respondents reported that they greatly increased their use of probiotics following CDI. Probiotics are microorganism-based products that have been proposed as a therapy to reconstitute disturbed gut microflora due to antibiotic use. The use of probiotics to prevent CDI is still controversial because of the conflicting findings across studies. Several systematic reviews and meta-analyses suggest that there is moderate evidence that specific probiotic strains and formulations may be effective and safe for primary prevention of CDI.Reference Goldenberg, Ma and Saxton 21 – Reference Evans and Johnson 23 Data show that those with RCDI have gut diversity that is significantly different from those with an initial CDI episode and more closely resemble normal controls.Reference Chang, Antonopoulos and Kalra 24 When separated from primary prevention studies of CDI, studies focusing on patients with previous CDI generally have been underpowered, showing little to no effect.Reference Evans and Johnson 23 Our finding that more than a third of respondents indicated increased use of probiotics following CDI episodes suggests that patients and/or their providers may not be aware of the lack of evidence regarding use of probiotics for secondary prevention.
Limitations to this study include a reliance on patient recall for events occurring from 6 months to 3.5 years prior. However, we found that changes in behavior were related to greater severity of diarrhea and to more days of inactivity, suggesting that recall of the effects of RCDI was long lasting. Another limitation was that many patients died prior to our being able to survey them. A recent study of utilization following RCDI found a 155% higher rate of 1-year mortality compared to matched patients who did not have CDI.Reference Kuntz, Baker, Kipnis, Li, Liu, Xie, Marcella and Escobar 12 We do not know how these individuals may have responded to the survey.
To our knowledge, this is the first study to survey patients about the impact of having had RCDI on subsequent prevention-related behaviors. It appears that RCDI may have resulted in some patients changing their behaviors related to hygiene and prevention. While some of these reported behaviors are appropriate (eg, hand washing), other behaviors are not supported by evidence of decreased risk. Providers should provide information (eg, pamphlets and/or waiting-room videos) about appropriate prevention behaviors to their CDI patients, and they should clarify that other behaviors (eg, eating out less) will not affect their risk of future illness but could negatively affect their quality of life.
ACKNOWLEDGMENTS
Financial support: The work was supported by funding from Chicago Area Patient Centered Outcomes Research Network (CAPriCORN; grant no. 1306-04737). The sponsor was not involved in the preparation, submission, or review of the manuscript.
Potential conflicts of interest: Dr Evans serves as a consultant for BioK+. Dr Mullane has grants from Merck, Astellas, and Actelion, and she serves as a consultant for Merck, Astellas, and Chimetrix. Dr Johnson is on the advisory boards of Synthetic Biologics and BioK+. Dr Gerding is a board member of Merck, Acetelion, and Bobiotix Summit, and he serves as a consultant for Minor, Groove, Binder (MGB); Sanofi Pasteur; DaVolterra; and Pfizer.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2017.208





