Current guidelines recommend the use of empiric vancomycin to cover methicillin-resistant Staphyloccoccus aureus (MRSA) for ill-appearing patients with a known history of MRSA colonization or infection, recent antibiotic exposure, complicated skin and soft-tissue infections, or recent hospitalizations.Reference Liu, Bayer and Cosgrove 1 – Reference Kalil, Metersky and Klompas 4 Patients with negative MRSA nasal surveillance swabs (referred to as nasal swabs in this document) have been shown to be at low risk for subsequent MRSA clinical infections.Reference Jinno, Chang and Donskey 5 – Reference Dangerfield, Chung, Webb and Seville 8 Because many acute-care facilities continue to obtain nasal swabs to identify patients colonized with MRSA, these data could be helpful when weighing decisions to initiate or stop anti-MRSA therapy for hospitalized patients. Studies evaluating the role of nasal swabs in guiding anti-MRSA therapy have not accounted for the 2–3-day turnaround time for results that precludes their use in guiding therapy before MRSA colonization status becomes available. Our primary objective was to determine the likelihood that patients with negative nasal swabs develop subsequent MRSA clinical infections during their current hospitalizations to assess whether vancomycin could be discontinued once nasal swabs results return negative. The secondary objectives were to assess days of vancomycin therapy that could potentially be avoided in the setting of negative nasal swabs and to identify risk factors to better identify patients with negative nasal swabs at risk to develop MRSA clinical infections during their hospital stay.
METHODS
Study Population
This retrospective cohort study was conducted at The Johns Hopkins Hospital, a 1,194-bed, tertiary-care medical center in Baltimore, Maryland. All patients admitted to intensive care units (ICUs) at our institution undergo MRSA nasal surveillance swabs at the time of ICU admission and weekly thereafter until ICU discharge. Nasal swabs are inoculated on selective and differential media to detect MRSA (BBL CHROMagar MRSA plates, Becton Dickinson, Franklin Lakes, NJ) as previously described.Reference Milstone, Carroll, Ross, Shangraw and Perl 9 For all patients admitted to any of 6 adult ICUs from December 1, 2013, to June 30, 2015, the following data were retrieved from our surveillance database (Theradoc, Hospira, Lakeforest, IL): (1) results of MRSA nasal swabs on ICU admission and weekly thereafter, (2) any available MRSA surveillance or clinical culture results prior to the current admission within 12 months, and (3) all MRSA-positive culture isolates during ICU admission. Each patient was reviewed from the time they had negative MRSA clinical cultures and nasal swabs until they had positive MRSA clinical cultures or nasal swabs in the ICU.
To identify the proportion of subsequent MRSA infections among all patients with negative nasal swabs, we excluded the following patients: (1) patients with positive MRSA surveillance or clinical cultures within 12 months prior to the current admission, and (2) patients who did not have nasal swabs obtained prior to MRSA infection during the current admission. MRSA infection was defined based on recovery of MRSA in clinical cultures and treatment by the clinical team.
Many patients who have nasal swabs are not suspected of having infections either at the time of ICU admission or later during the same hospitalization. Thus, they are not the population of interest when determining the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of nasal swabs in predicting subsequent MRSA infection. To identify patients “at risk” for MRSA infections, patients with nasal swabs at admission were reviewed for initiation of vancomycin at any point during the same admission after their index ICU nasal swab. Initiation of vancomycin was used as a proxy for suspicion of clinical MRSA infection. As our institution generally used only vancomycin for empiric coverage of MRSA. Patients who received vancomycin for non-MRSA infections in which vancomycin was indicated (eg, oxacillin-resistant coagulase-negative Staphylococcus infection, anaphylactic penicillin allergy, etc) were excluded from sensitivity, specificity, PPV, and NPV estimates.
Assessment of Potentially Avoidable Vancomycin Use
The indication for vancomycin use and the duration of vancomycin therapy were determined. Because results of nasal swabs are generally not available until day 3 after they are obtained, only days of vancomycin therapy from day 4 or later (after nasal swabs were obtained until vancomycin was discontinued) were included when determining the duration of vancomycin therapy. Both vancomycin that was continued in the presence of a negative nasal swab (and no prior clinical cultures growing MRSA) and vancomycin that was started during the same admission, when it was known that the patient had had a negative nasal swab (and no prior clinical cultures growing MRSA), were included.
Case-Control Study
We conducted a matched case-control study to identify risk factors for patients with negative nasal swabs who subsequently developed MRSA infections during their hospitalization. For the case-control study, independent adjudication of patients with MRSA growing in clinical cultures who received MRSA-targeted therapy by the clinical team was performed by 3 infectious diseases physicians (D.C., P.D.T., and S.E.C.). True infection was defined as (1) presence of MRSA bacteremia; (2) MRSA isolated in sputum cultures in conjunction with signs and symptoms of pneumonia and presence of an infiltrate on chest imaging; (3) MRSA isolated from cerebral spinal fluid; MRSA isolated from intra-abdominal fluid; and/or (4) MRSA isolated in cultures from skin, soft tissue, bone, or joints with signs and symptoms of infection. Only patients who had been determined to have true infections were included as case patients. Each case patient was matched to 3 controls. Controls consisted of patients with negative nasal swabs who did not develop MRSA infections during their hospitalizations. Controls were recruited randomly from the same ICUs as case patients. Controls needed to be hospitalized for at least the same number of days as the time from when a case patient had a MRSA surveillance culture to the time when MRSA clinical cultures developed. For example, if the case patient developed a MRSA infection 10 days after the MRSA nasal swab was obtained, the control needed to be in the hospital at least 10 days after his or her negative nasal swab. For case patients, time at risk was defined as the period from when nasal swabs were obtained to the time MRSA was recovered from clinical cultures. For controls, time at risk was the period from when nasal swabs were obtained until hospital discharge.
The following baseline demographic and clinical data were collected from electronic medical records for all case patients and controls: preexisting medical conditions, APACHE II scores, hospital location prior to ICU admission (emergency department versus hospital ward), mechanical ventilation, central venous catheter, surgery, other invasive procedures (ie, angiography, embolization, bronchoscopy, or hemodialysis), and all intravenous and oral antibiotic therapy prior to development of MRSA infection in the current admission. The Johns Hopkins University Institutional Review Board approved this study and waived informed consent.
Statistical Analysis
The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of nasal swabs in predicting subsequent MRSA infection were calculated among patients who were initiated on vancomycin as described previously. Sensitivity was defined as the probability the nasal swab was positive when a MRSA infection occurred at any point until ICU discharge. Specificity was defined as the probability the nasal swab was negative when no MRSA infection occurred at any point until ICU discharge. The positive predictive value was defined as the ability of a positive nasal swab test to predict subsequent MRSA infection during the ICU stay. The negative predictive value was defined as the ability of a negative nasal swab test to correctly predict no subsequent MRSA infection during the ICU stay.
Matched case-control analysis was performed using conditional logistic regression to identify independent risk factors associated with MRSA infection. Results were presented as unadjusted odds ratios (ORs), with corresponding 95% confidence intervals (CIs). Two-sided P values<.05 were considered statistically significant. Data analysis was performed using Stata version 13.0 software (StataCorp, College Station, TX).
RESULTS
Between December 2013 and June 2015, 12,215 patients had nasal swabs in 6 adult ICUs. After the exclusion of 260 encounters with patients with prior MRSA infection or colonization in the previous year and 73 encounters with patients with no nasal swabs obtained prior to MRSA infection during admission, 11,441 patients (96.3%) had negative nasal swabs and 441 patients (3.7%) had positive nasal swabs. Overall, 403 patients had positive nasal swabs on ICU admission and 38 patients had positive nasal swabs on subsequent sampling (Figure 1). Of 38 patients, 5 (13.2%) with subsequent positive nasal swabs went on to develop MRSA infections. The median numbers of nasal swabs obtained during admission in the negative swab and positive swab groups were 1 (range, 1–18; interquartile range [IQR], 1–1) and 1 (range, 1–22; IQR, 1–2), respectively. Among 441 patients with positive nasal swabs, 99 patients (22.4%) had subsequent isolation of MRSA in clinical cultures, and 65 of these patients (14.7%) were treated for MRSA by the clinical team. Among the 11,441 patients with negative nasal swabs, 49 had subsequent isolation of MRSA in clinical cultures, and 25 of these patients (0.22%) were treated for MRSA by the clinical team.
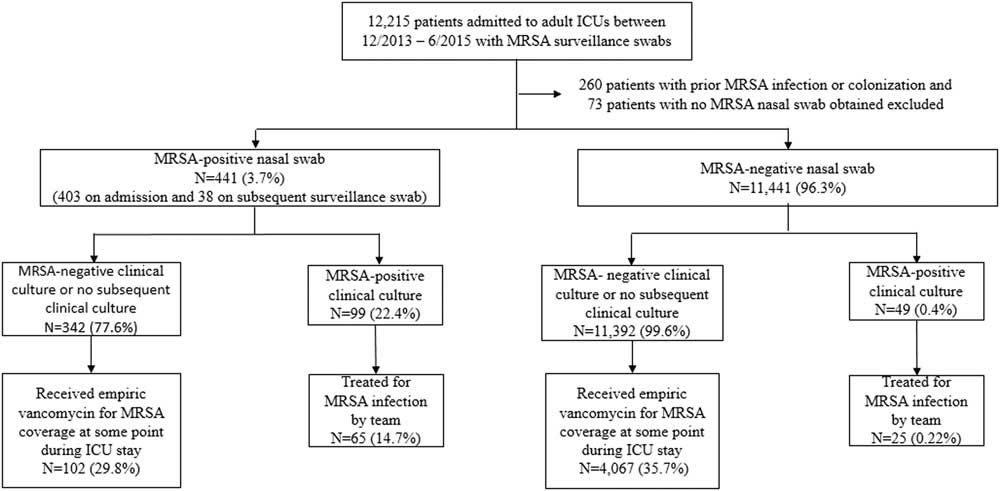
FIGURE 1 Chronological sequence of MRSA surveillance nasal swab, clinical culture, and vancomycin prescription.
Of the 11,392 patients with negative nasal swabs and no evidence of subsequent MRSA infection or colonization, 4,133 patients (36.3%) received vancomycin, either upon admission to the ICU or later during their ICU stay. Moreover, 63 patients received vancomycin for bacterial infections other than MRSA: oxacillin-resistant coagulase-negative staphylococci, ampicillin-resistant enterococci, Streptococcus pneumoniae, Corynebacterium spp, or Propionibacterium acnes. Furthermore, 3 patients had a history of anaphylaxis to β-lactams and were excluded from the analysis, leaving 4,067 patients who received vancomycin. Of the 441 patients with positive nasal swabs, 102 received empiric vancomycin in the ICUs.
When assessing 4,317 patients who received vancomycin for suspected MRSA infection, 65 patients in the positive swab group and 25 patients in the negative swab group had a MRSA infection based on judgment of the treating clinician. The sensitivity and specificity of the nasal swab screening test for subsequent MRSA infection was 72.2% (95% CI, 61.6%–80.9%), and 96.8% (95% CI, 96.2%–97.3%), respectively (Table 1). The PPV and NPV were 32.3% (95% CI, 26.0%–39.3%) and 99.4% (95% CI, 99.1%–99.6%), respectively.
TABLE 1 Sensitivity, Specificity, Positive Predictive Value, and Negative Predictive Value of Methicillin-Resistant Staphylococcus aureus (MRSA) Nasal Swabs in Detecting MRSA Infections Among Patients Considered to be at Risk for MRSA Infections by the Treating ClinicianFootnote a

NOTE. CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value.
a Sensitivity, 72.2% (95% CI, 61.6%–80.9%); PPV, 32.3% (95% CI, 26.0%–39.3%), specificity, 96.8% (95% CI, 96.2%–97.3%), NPV, 99.4% (95% CI, 99.1%–99.6%).
Of the 4,067 patients receiving vancomycin, 3,343 patients (82.2%) received vancomycin empirically upon ICU admission and 745 (18.3%) patients were initiated on vancomycin at some point in the ICUs after it was known they had a negative nasal swab. Among the 3,343 patients who received vancomycin upon ICU admission, vancomycin was not stopped by day 4 in 686 patients (20.5%). Thus, 2,643 days of vancomycin were administered for a median of 2 additional days (IQR, 1–4 days). Furthermore, 306 of these 686 patients also went on to receive an additional course of vancomycin during an ICU stay, accounting for an additional 1,465 vancomycin days, for a median of 3 days (IQR, 2–6 days). The 745 patients known to have negative nasal swabs during their current hospitalization who were initiated on vancomycin sometime after admission accounted for 2,573 days of vancomycin, for a median of 3 days (IQR, 1–4 days) of vancomycin. Of these patients, 126 received an additional course of vancomycin, accounting for an additional 683 vancomycin days for a median of 4 days (IQR, 2–7) (Figure 2). The total days of vancomycin after 3 days from the first negative nasal swab were 7,364 days in 4,067 patients.
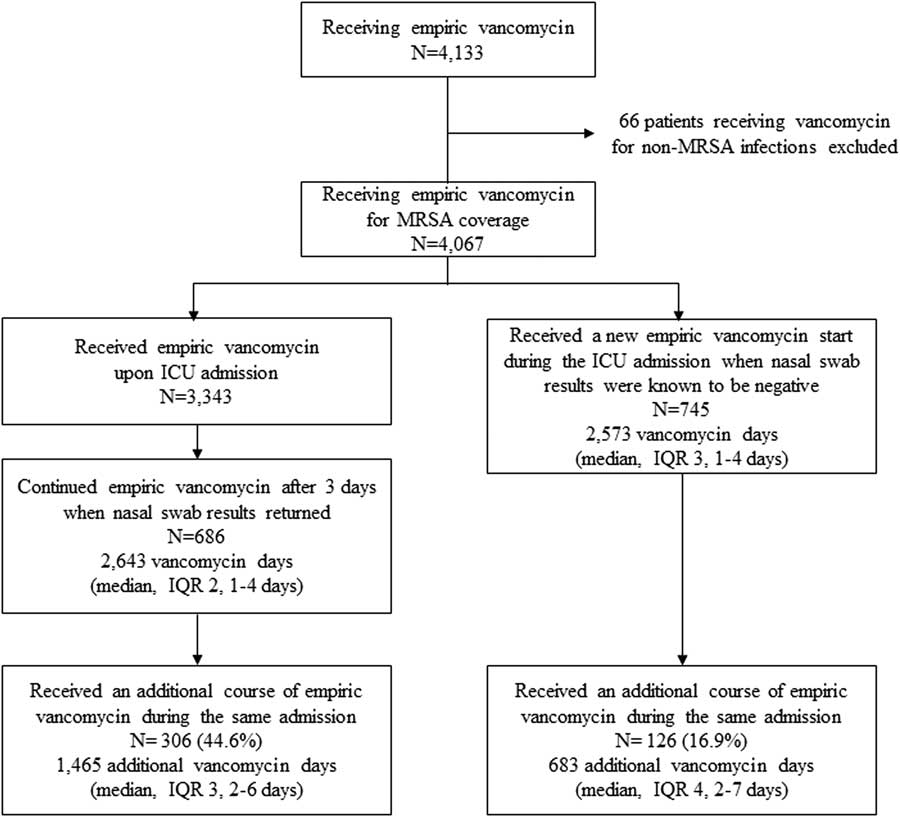
FIGURE 2 Receipt of vancomycin among patients with negative MRSA nasal surveillance swabs.
Among 14 negative nasal swabs with subsequent MRSA infections based on review by 3 infectious diseases physicians, the median duration of time from a negative nasal swab encounter to a MRSA infection was 6 days (IQR, 1–11 days; range, 1–25 days). Furthermore, 6 patients had MRSA bacteremia, 5 patients had hospital-acquired MRSA pneumonia, and 3 patients had surgical site infections. Although higher proportions of case patients had solid organ transplants, chronic organ dysfunction, and invasive procedures (eg, central-line placement, or intubation), the matched case-control univariate analysis did not identify any significant risk factors associated with subsequent MRSA infection (Tables 1 and 2).
TABLE 2 Demographic Data, Duration of Hospitalization and Time at Risk of Patients With Negative Methicillin-Resistant Staphylococcus aureus (MRSA) Nasal Swabs Who Did and Did Not Develop MRSA Infections During the Same Hospitalization
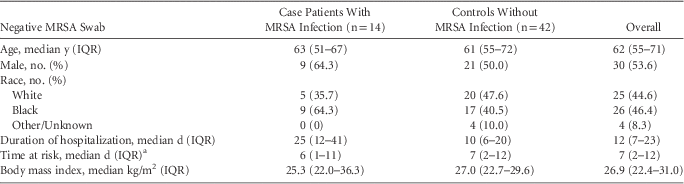
NOTE. IQR, interquartile range.
a Time at risk, for case patients was defined as the time from MRSA surveillance cultures obtainment to the time when MRSA clinical cultures were obtained, for controls defined as the time when MRSA surveillance cultures were obtained until hospital discharge.
DISCUSSION
In this study, among patients with negative MRSA nasal surveillance swabs, well under 1% went on to develop a MRSA infection during the same hospitalization. The suboptimal sensitivity and low PPV but high specificity and high NPV of nasal swabs in predicting subsequent infection observed in our study is in agreement with most previous reports.Reference Jinno, Chang and Donskey 5 , Reference Harris, Furuno and Roghmann 6 , Reference Pirracchio, Mateo and Raskine 10 , Reference Chan, Dellit and Choudhuri 11 The poor performance of nasal swabs in predicting subsequent risk of lower respiratory tract infection or bloodstream infection caused by MRSA was reported in a study that took place in a medical ICU with a higher prevalence of MRSA infections.Reference Sarikonda, Micek, Doherty, Reichley, Warren and Kollef 12 In this study, subsequent MRSA infection occurred in similar proportions of patients with and without MRSA nasal colonization (27.4% vs 22.7%),Reference Sarikonda, Micek, Doherty, Reichley, Warren and Kollef 12 in contrast to our study (14.7% vs 0.22%). However, 31% of patients did not have nasal swabs performed upon admission to the medical ICU; thus, it is possible that patients with MRSA colonization were not detected and isolated and acted as a reservoir for transmission.
The low overall number of MRSA cases in our study population drives a high NPV; thus, no diagnostic test will add much incremental diagnostic value to chance alone. Nonetheless, the perceived risk for MRSA by clinicians is high given the described high prevalence of MRSA in our geographic area. Many clinical guidelines recommend that patients being admitted to ICUs for infection or with subsequent hospital-acquired infections generally should be treated with vancomycin to cover MRSA. Despite this low probability of MRSA infection, vancomycin was continued or started for 36% of patients with symptoms indicating a possible bacterial infection after their providers knew their patients had negative nasal swabs.
The security of having a laboratory test to identify patients at low risk is likely to be helpful in guiding cessation of therapy and avoiding new restarts of therapy in the face of the fear of MRSA that has evolved over the past decade. Although not the focus of this investigation, 14.7% of patients with MRSA colonization did go on to have a subsequent MRSA infection, demonstrating the increased risk in this group of patients and arguably justifying empiric vancomycin.
Vancomycin overuse has been demonstrated in several studies. In a study conducted at a university teaching hospital in Brazil, ~70% of patients continued to receive vancomycin at 72 hours despite no obvious indication for its use.Reference Junior, Correa, Marra, Camargo and Pereira 13 The use of a prediction rule consisting of nasal swab results and clinical risk factors for patients with sepsis of unclear origin was estimated to reduce empiric vancomycin use by 29%.Reference Jinno, Chang and Donskey 5 Efforts to improve vancomycin prescribing practices should focus both on unnecessary vancomycin initiation as well as prolonged durations of therapy. This is particularly important because vancomycin use is associated with subsequent isolation of drug-resistant organisms, including vancomycin-resistant EnterococcusReference Carmeli, Samore and Huskins 14 and vancomycin-intermediateReference Lodise, Graves and Evans 15 , Reference Lodise, Miller and Graves 16 or resistant Staphylococcus aureus,Reference Tenover 17 , Reference Tiwari and Sen 18 and with adverse drug events, most notably nephrotoxicity.Reference van Hal, Paterson and Lodise 19
In our matched case-control study, we were unable to identify any risk factors associated with MRSA infection among patients with negative nasal swab encounters. This analysis was likely limited by the small sample size of patients with negative nasal swabs who subsequently developed MRSA infections (n=14). The median time from a negative swab to clinical infection with MRSA was 6 days. This may be a consequence of improper MRSA surveillance swab acquisition not detecting existing nasal colonization or MRSA colonization at other non-nares sites. We did not perform a broth enrichment step, which could have increased MRSA detection by 12%–26%.Reference Nahimana, Francioli and Blanc 20 , Reference van Loo, van Dijk, Verbakel-Schelle and Buiting 21 However, this approach is resource intensive, and currently, no standardized MRSA screening protocol has been recommended.Reference Fagan, Jenkins, Walton and James 22 , Reference Marlowe and Bankowski 23 No extranasal surveillance swabs were performed in our ICUs, mimicking general practice in most US ICUs. Thus, it is unknown whether obtaining MRSA surveillance swabs from other body sites would have led to earlier identification of the MRSA status of these 14 patients. It has been previously shown that nasal swab cultures missed only 2% of patients colonized with MRSA in sites other than the nares.Reference Baker, Brecher, Robillard, Strymish, Lawler and Gupta 24
Our study has several limitations. First, this study was conducted at a single tertiary referral center with intensive infection control practices, such as daily chlorhexidine bathing of patients in the ICU,Reference Chen, Li, Li, Wu and Zhang 25 contact precautions for patients with MRSA colonization with a high compliance rate by healthcare workers, hand hygiene compliance in >90% of episodes, and good compliance with obtaining nasal swabs in the ICU. All of these likely play a role in the low rate of MRSA transmission among hospitalized patients in our institution. In addition, we have a robust antibiotic stewardship program (ASP) that has been in place since 2001, and critical care clinical pharmacy specialists in all adult ICUs provide feedback to teams about antibiotic selection, including prompting for cessation of vancomycin. The rate of vancomycin days per 1,000 patient days at our institution has historically been slightly lower than that of similar institutions.Reference Polk, Hohmann, Medvedev and Ibrahim 26 Thus, our estimates of days of vancomycin that might be avoided could be lower than in institutions without ASPs.
Second, we did not use rapid molecular tests to detect MRSA nasal colonization. The use of real-time polymerase chain reaction (PCR) for MRSA detection can reduce the turnaround time for MRSA colonization status to as little as 4 hours.Reference Wu, Jeyaratnam, Tosas, Cooper and French 27 In our cohort, if we had used a test that resulted within 1 day, the number of potentially avoidable vancomycin days would have increased from 7,364 days to 9,000 days during the 19-month study period.
Third, our institution is in a geographic area with a high prevalence of MRSA in the community,Reference Furuno, Hebden and Standiford 28 which might increase the likelihood that patients are colonized with MRSA at sites other than the nares. Thus, our results may not be generalizable to hospitals with a lower prevalence of MRSA in the community where the risk of missing baseline MRSA colonization may be lower. However, in such a setting, the risk of missing a case of MRSA infection using negative nasal swabs as a guide may actually be lower. Finally, we reviewed cases retrospectively, and clinical data may have informed decisions to initiate vancomycin that were not available in the medical record.
In conclusion, for institutions that obtain MRSA nasal swabs as part of infection control practices and have a low prevalence of MRSA transmission, negative MRSA nasal swabs can be used to identify ICU patients with a low risk of MRSA infection in whom initial empiric vancomycin can be stopped and in whom subsequent empiric vancomycin starts can be avoided.
TABLE 3 Univariable Analysis of Potential Risk Factors for Methicillin-Resistant Staphylococcus aureus (MRSA) Acquisition for Patients With Negative Nasal Swabs Who Did and Did Not Develop MRSA Infections During the Same Hospitalization
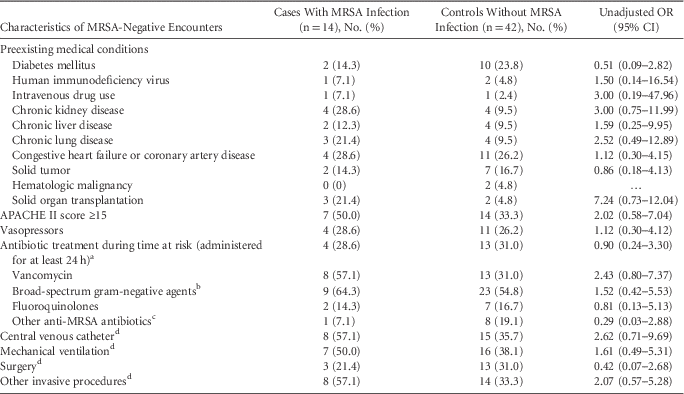
NOTE. OR, odds ratio; CI, confidence interval.
a Time at risk, for case patients, was defined as time from when MRSA surveillance cultures obtained to the time when MRSA clinical cultures were positive, for controls defined as time from when MRSA surveillance cultures obtained until hospital discharge.
b Piperacillin/tazobactam, ceftriaxone, cefepime, aztreonam, carbapenems.
c Trimethoprim-sulfamethoxazole, clindamycin, doxycycline, linezolid, ceftaroline, daptomycin, tigecycline.
d During the current hospitalization from the time of admission to the time at risk.
ACKNOWLEDGMENTS
Financial support: No financial support was provided relevant to this article.
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.






