Introduction
The bacterium Deinococcus radiodurans is known for its ability to endure severe environmental stresses. Remarkably, it survives in the vegetative state, rather than as specialized structures such as endospores. Populations of its cells can tolerate 5 kGy of gamma radiation and over 400 J m−2 of extremely DNA damaging monochromatic UV-C (254 nm) radiation without measurable loss of viability (Battista, Reference Battista1997). In addition, it can survive desiccation for prolonged periods under very low-relative humidity and even ultra-high vacuum (Bauermeister et al., Reference Bauermeister, Moeller, Reitz, Sommer and Rettberg2011). Resistance to these forms of stress is mainly associated with this bacterium's efficient DNA repair and with antioxidant systems that protect cellular components from oxidative damage (Mattimore and Battista, Reference Mattimore and Battista1996; Fredrickson et al., Reference Fredrickson, Li, Gaidamakova, Matrosova, Zhai, Sulloway, Scholten, Brown, Balkwill and Daly2008; Slade and Radman, Reference Slade and Radman2011).
In astrobiology studies, D. radiodurans can be used as an indicator of the limits of life as we know it under different simulation conditions. It has been applied to understand the response of living cells to the environment on the surface of Mars (Diaz and Schulze-Makuch, Reference Diaz and Schulze-Makuch2006; Pogoda de la Vega et al., Reference Pogoda de la Vega, Rettberg and Reitz2007), for example, and outer space (Paulino-Lima et al., Reference Paulino-Lima, Pilling, Janot-Pacheco, de Brito, Barbosa, Leitao and Lage2010; Abrevaya et al., Reference Abrevaya, Paulino-Lima, Galante, Rodrigues, Mauas, Corton and Lage2011), which is the focus of this work. Studies of this type have been conducted with many different life forms and biomolecules on laboratory facilities and even at the real space environment: aboard the International Space Station, on rocket flights, and on satellites (Horneck et al., Reference Horneck, Klaus and Mancinelli2010; Olsson-Francis and Cockell, Reference Olsson-Francis and Cockell2010). An underexplored but relatively accessible extraterrestrial analogue environment is the Earth's stratosphere, a Mars-like setting which can be reached by high-altitude balloons (DasSarma et al., Reference DasSarma, Laye, Harvey, Reid, Shultz, Yarborough, Lamb, Koske-Phillips, Herbst, Molina, Grah, Phillips and DasSarma2017; Khodadad et al., Reference Khodadad, Wong, James, Thakrar, Lane, Catechis and Smith2017; Pulschen et al., Reference Pulschen, de Araujo, Carvalho, Cerini, Fonseca, Galante and Rodrigues2018). These contributions are of relevance to placing constraints on planetary surface habitability in relation to the stellar radiation environment (and its evolution through time), informing planetary protection protocols and policies, and allowing a critical evaluation of panspermia hypothesis.
UV radiation is one of the most important aspects of these simulations, often being the main inactivating factor. On Earth, ozone photochemically formed from molecular oxygen in the stratosphere absorbs and effectively blocks the more energetic, damaging solar radiation wavelengths of UV-C (200–280 nm) and largely attenuates UV-B (280–320 nm), while UV-A (320–400 nm) is preserved. Mars lost most of its atmosphere through geological time (Brain and Jakosky, Reference Brain and Jakosky1998), and the entire UV spectrum with wavelengths above 200 nm reaches the surface of the planet, which has severe biological consequences on exposed areas (Cockell et al., Reference Cockell, Catling, Davis, Snook, Kepner, Lee and McKay2000). Nevertheless, the protecting effect of shading provided by soil, dust and salts over microbial cells has been demonstrated in Mars simulations, which can raise survivability significantly (Diaz and Schulze-Makuch, Reference Diaz and Schulze-Makuch2006; Osman et al., Reference Osman, Peeters, La Duc, Mancinelli, Ehrenfreund and Venkateswaran2008; Smith et al., Reference Smith, Schuerger, Davidson, Pacala, Bakermans and Onstott2009). In interplanetary space, another setting of astrobiological interest, conditions are even harsher.
The outer space environment is somewhat harder to simulate in laboratory experiments than planetary ones, especially in terms of its radiation environment. Figure 1 shows the Sun's ultraviolet spectrum outside Earth's atmosphere. It is continuous, extending much further bellow UV-C, exhibiting a high-intensity peak at 121.6 nm which is the hydrogen Lyman alpha emission line. The high-energy vacuum ultraviolet (VUV) wavelengths (λ < 200 nm) are strongly absorbed by gasses in the atmosphere and are not able to travel through air. Simulations on ground-based facilities must be conducted in chambers at very low pressures and use specialized VUV photon sources such as hydrogen lamps (Paulino-Lima et al., Reference Paulino-Lima, Pilling, Janot-Pacheco, de Brito, Barbosa, Leitao and Lage2010), excimer lamps (Kawaguchi et al., Reference Kawaguchi, Yang, Kawashiri, Shiraishi, Takasu, Narumi, Satoh, Hashimoto, Nakagawa, Tanigawa, Momoki, Tanabe, Sugino, Takahashi, Shimizu, Yoshida, Kobayashi, Yokobori and Yamagishi2013; Zvereva et al., Reference Zvereva, Kirtsideli, Benken, Saifitdinova, Galkina, Parfenov, Tarasenko and Kabanov2015), lasers (Sarantopoulou et al., Reference Sarantopoulou, Gomoiu, Kollia and Cefalas2011, Reference Sarantopoulou, Stefi, Kollia, Palles, Petrou, Bourkoula, Koukouvinos, Velentzas, Kakabakos and Cefalas2014) and synchrotron beamlines (Paulino-Lima et al., Reference Paulino-Lima, Pilling, Janot-Pacheco, de Brito, Barbosa, Leitao and Lage2010; Abrevaya et al., Reference Abrevaya, Paulino-Lima, Galante, Rodrigues, Mauas, Corton and Lage2011). In particular, synchrotron facilities are useful for photobiological investigations in this range for being able to provide very low-wavelength photons while maintaining a high flux (Ito, Reference Ito, Sweet and Woodhead1989).
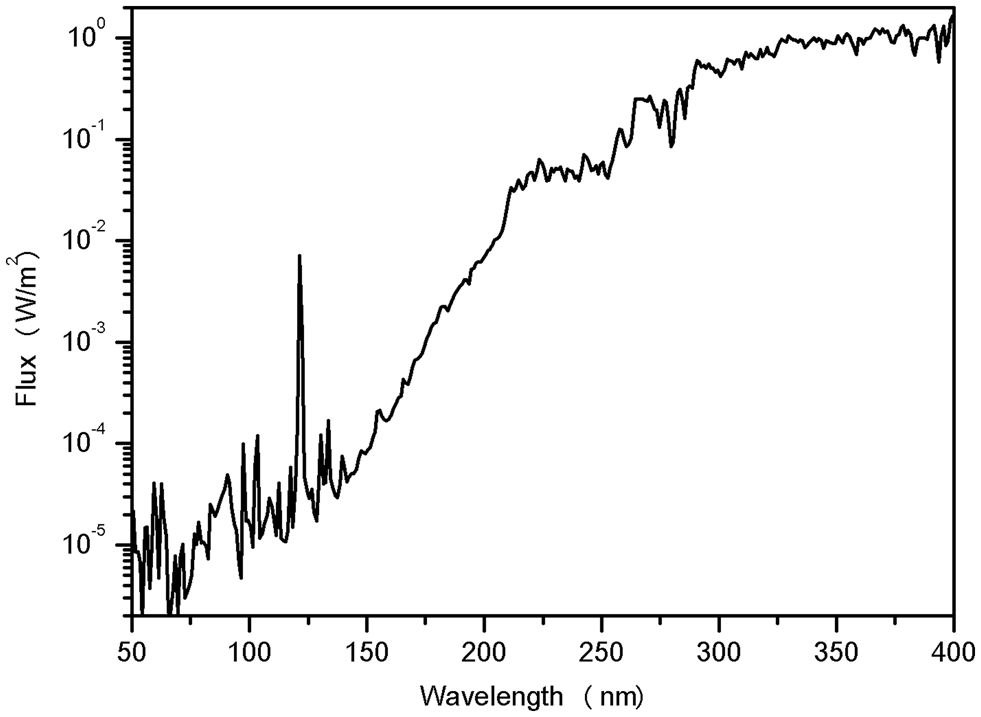
Fig. 1. Ultraviolet solar spectrum outside Earth's atmosphere. The solar flux was obtained with the software Solar 2000 v2.36 with database S2K + ASTM490, with daily solar spectra from 1999–2010 (covering a full 11-year solar cycle). The variation of the data throughout the years was smoothed to an averaged curve. The prevailing peak in the VUV at 121.6 nm is the hydrogen Lyman alpha emission line.
After the simulations, measuring the viability of the cells (their ability to continually multiply), using the traditional plating in solid medium and enumeration of colony forming units (CFU), is a useful, but limited methodology. This approach is not capable of discriminating the basic mechanisms of inactivation and how they contribute to ultimately kill the organism. In this work, specific molecular probes were allied with flow cytometry techniques for a more in-depth look. With this method, whole populations can be studied from cells that are passed orderly through a capillary flow channel where they are analysed by a laser beam one by one. A membrane damage probe (propidium iodide (PI)) and a probe for oxidative stress (dihydrorhodamine 123 (DHR)) were used.
A combination of these flow cytometry techniques with traditional microbiological methods was applied to study the biological response of a model extremotolerant bacterium (D. radiodurans) to a condition of astrobiological interest: a surface not protected by any atmosphere and exposed to the solar radiation in the interplanetary space, such as on the Moon, an asteroid or the outside of a spacecraft. At the Brazilian Synchrotron Light Laboratory (LNLS – CNPEM, Campinas, Brazil), the toroidal grating monochromator (TGM) beamline was chosen for this outer space simulation. It produces radiation in the VUV range, and samples can be exposed inside an ultra-high vacuum chamber (Cavasso Filho et al., Reference Cavasso Filho, Homen, Fonseca and Naves de Brito2007). The simulation's conditions and a comparison to the space environment are summarized in Table 1.
Table 1. Physical conditions in interplanetary space, low Earth orbit and inside the ultra-high vacuum chamber at the TGM beamline

The UV region of the continuum solar spectrum found in space can be seen in Fig. 1. Modified from Nicholson et al. (Reference Nicholson, Munakata, Horneck, Melosh and Setlow2000) and Paulino-Lima et al. (Reference Paulino-Lima, Pilling, Janot-Pacheco, de Brito, Barbosa, Leitao and Lage2010).
a Value depends on orientation and distance to the Sun.
b See Fig. 1.
c Depends on outgassing of the spacecraft.
Materials and methods
D. radiodurans (R1 wild type strain) was routinely grown on liquid TGY medium (tryptone, 10 g l−1; glucose, 2 g l−1; yeast extract, 6 g l−1) at 30°C under orbital agitation. Stationary phase cells were harvested from the cultures by centrifugation and washed twice with 0.9% NaCl solution. For the experiments at the TGM beamline, the suspension of washed cells was deposited on glass slides as a series of 2 µl droplets (containing about 106 cells) and air-dried inside a laminar flow hood to avoid contamination. A parallel set of samples prepared like these were gold-sputtered before imaging under ultra-high vacuum in a scanning electron microscope (FEI Quanta 650 FEG) to enable the identification of cell clumps and other shading materials that could have formed during drying. The glass slides were attached with copper tape to the sample holder and placed inside the beamline vacuum chamber. The pressure in the chamber was brought down to ultra-high vacuum (10−6 Pa, similar to low-earth orbit, Table 1) by the combined action of a mechanical pump and a turbomolecular pump. The temperature of the samples was not controlled, so they remained at room temperature (~295 K).
The beamline was configured with its diffraction grating at zero-order, producing a broad range spectrum that included a higher energy X-ray portion, which had to be attenuated to ensure a better fidelity to the Solar spectrum (Paulino-Lima et al., Reference Paulino-Lima, Pilling, Janot-Pacheco, de Brito, Barbosa, Leitao and Lage2010). This was performed by another chamber midway through the beamline containing neon gas at a higher pressure, maintained by a differential pumping system. The gas effectively filters radiation with energies over 21.5 eV (λ < 57.6 nm), removing the soft X-ray portion of the beamline spectrum while preserving most of the VUV (Cavasso Filho et al., Reference Cavasso Filho, Homen, Fonseca and Naves de Brito2007; Paulino-Lima et al., Reference Paulino-Lima, Pilling, Janot-Pacheco, de Brito, Barbosa, Leitao and Lage2010). Measurements of the photon flux were taken using a calibrated photodiode (IRD AXUV100). Photos of the experimental setup are presented in Fig. 2.

Fig. 2. Experimental setup at the LNLS synchrotron TGM beamline. The samples are shown placed on glass slides attached to the sample holder that goes inside the ultra-high vacuum chamber.
The droplets were successively irradiated by being placed in the path of the beam for different amounts of time, controlled by a shutter. Its positioning over the samples could be easily done by direct observation, by placing a glass filter on the path of the beam, letting only visible, innocuous light, pass. The duration of the exposures needed for a given final fluence was calculated from the intensities measured by the photodiode.
After the successive irradiation of all the samples, the chamber was vented and the glass slides were recovered from the sample holder. Cells were resuspended from the glass slides by successively pumping with a micropipette 20 µl of 0.9% NaCl solution over the dried droplets and transferring to a microtube for a final volume of 1 ml. This suspension was serially diluted and plated on solidified TGY medium (with agar, 1.5%) for the counting of CFU. Survival was quantified by N/N 0, where N is the recovered number of CFU (adjusted by the dilution factor) after the simulation and N 0 is the number of CFU of ultra-high vacuum-exposed, non-irradiated control samples (‘0 J m−2’). Additional controls for the high-vacuum treatment, not used for the survival calculations, were non-desiccated cells sampled directly from the culture.
For the flow cytometry assays, the cells were stained with specific probes. For the quantification of membrane damage, PI was used. PI is a fluorescent molecule with high affinity for nucleic acids, but it is unable to cross the cytoplasmatic membrane of an intact cell. Only when the membrane is somehow damaged, PI can interact with the DNA and stain the interior of the cell. Based on this, using the suitable laser line of a flow cytometer (within the probe's excitation range), fluorescent (damaged) the cells can be quantitatively distinguished from intact, non-fluorescent ones (Baatout et al., Reference Baatout, De Boever and Mergeay2005).
The PI staining was performed with 0.15 µl ml−1 of PI stock solution (20 mM in DMSO, Life technologies) followed by incubation in the dark for 15 min. The suspension was read by the 488 nm laser line of a BD FACSCanto II flow cytometer (Becton Dickinson), acquired using BD FACSDiva software. The fluorescence signal of the probe was collected at the appropriate channel and 10 000 events (particles/cells counted) were recorded. The results were analysed by the software FCS Express v. 3 (De Novo Software). The fraction of the cells that had higher fluorescence intensity in the region PI emits was considered ‘stained’. The proportion was determined in relation to the cells read as less intense, equivalent to ‘non-stained’, or intact, by the flow cytometer. These populations were differentiated based on the staining pattern of non-desiccated intact cells obtained directly from the culture, and compared with dead cells treated with 70% membrane-permeabilizing ethanol solution (Fig. 3).
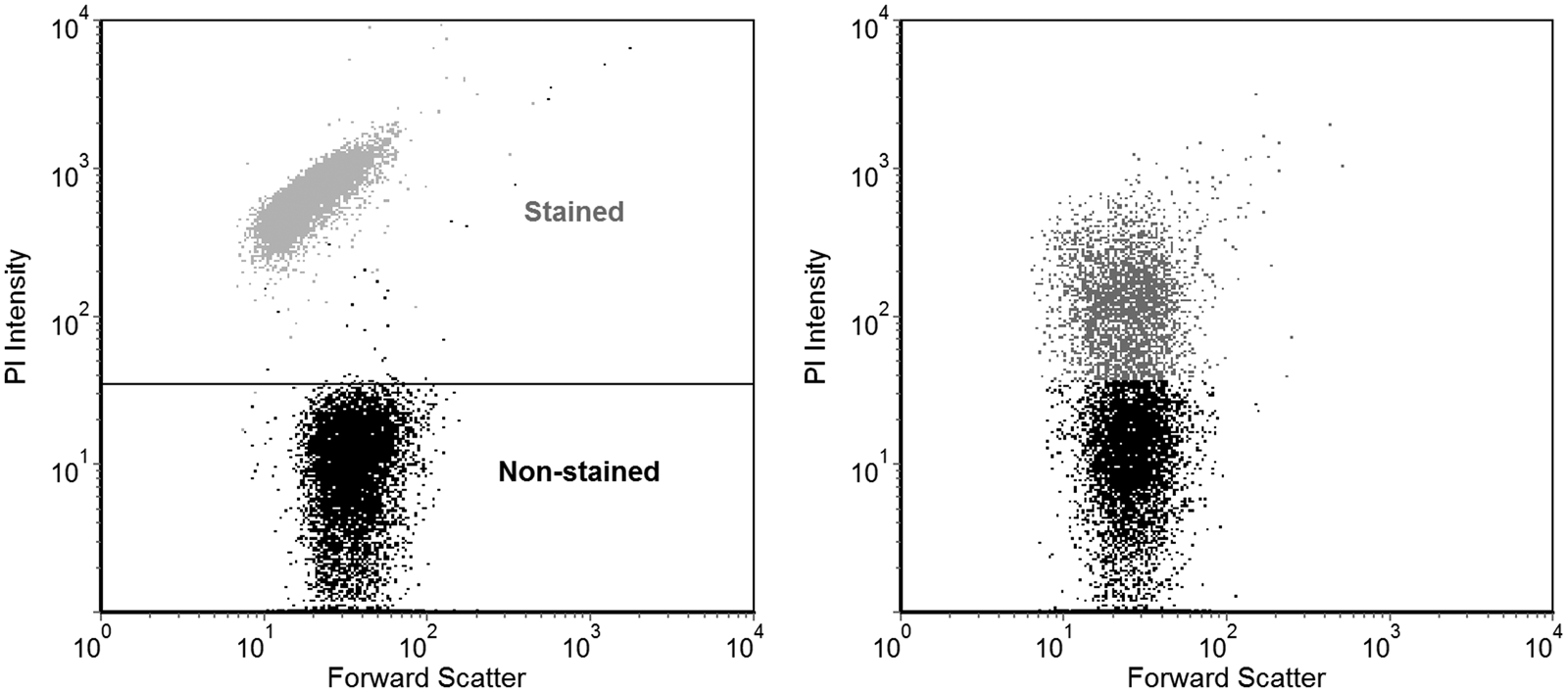
Fig. 3. Example of flow cytometry data. Each dot represents the measurement of the fluorescence and forward scattering parameters of a single scanned cell. Only the fluorescence values were used for the analyses. A control population of cells from the culture, with added PI, was used to define the threshold fluorescence intensity above which the cells can be considered stained, as can be seen in the lower portion of the plot on the left. As an example, cells completely permeabilized with 70% ethanol are shown overlaid on this plot, exhibiting much higher fluorescence intensity. On the right, a population of partially damaged cells from a vacuum-exposed non-irradiated sample is shown, where a proportion of them has increased in fluorescence beyond the previously defined threshold and thus could be counted as stained.
The other probe tested, used as an oxidative stress marker, was DHR which itself is permeable to cellular membranes, but non-fluorescent. The generation of reactive oxygen species within the cell, such as the ones formed upon UV irradiation, converts this compound to its oxidized form, rhodamine 123. This intracellularly-generated molecule is fluorescent, and its intensity in a population can also be measured by flow cytometry (Wilhelm et al., Reference Wilhelm, Vytasek, Ostadalova and Vajner2009). This probe has already been used to measure oxidative stress induced by UV radiation in D. radiodurans (Krisko and Radman, Reference Krisko and Radman2010).
Suspensions of cells to be assayed for oxidative stress were initially stained with 4.33 µl ml−1 of DHR stock solution (2 mg ml−1 in DMSO, Sigma) to a final concentration of 25 µM. After being incubated for 15 min in the dark, these samples were then deposited on the glass slides (2 µl volumes), taken to the vacuum chamber, and irradiated at the beamline as described for the other samples. Afterwards, being recovered in saline solution, their fluorescence (at the appropriate green channel) was measured by the flow cytometer in a similar manner as the PI staining was, except that this data was used as a more qualitative analysis. Only the mean fluorescence intensity was used, not the proportions of differently stained cells.
The data presented was obtained from triplicates of independently irradiated droplets, and the error bars denote standard deviations of the means. PI staining tests were performed with the same aliquots used for cell survival quantification by CFU counting, while the DHR experiments, with cells stained prior to desiccation, were performed with a separate set of samples used just for this analysis.
Results
For the experiments, suspensions of D. radiodurans cultures washed with NaCl 0.9% solution were deposited on top of glass slides and air dried before being taken to the ultra-high vacuum chamber. Scanning electron microscopy (SEM) imaging of the samples prepared in this manner showed that, after drying, formation of cells clumps and salt crystals had occurred (Fig. 4). Since VUV is highly interacting, this could have protected a small subset of the bacterial population from the radiation, as was confirmed by the results presented below. Nevertheless, most of the cells should have been at least partially exposed.

Fig. 4. D. radiodurans cells over the glass surface used for the TGM experiments visualized by a FEI Quanta 650 FEG scanning electron microscope. These samples were prepared in the same manner as the ones subjected to the interplanetary space simulation, except they were gold-sputtered before imaging under ultra-high vacuum. Cell clusters and salt deposits can be seen in the images.
The results of the CFU-counting and flow cytometry experiments with D. radiodurans under the simulated interplanetary space conditions are all shown in Fig. 5 for a more direct comparison between the factors on a fluence basis. The initial data point, ‘N 0’, represents vacuum-exposed, non-irradiated cells recovered from the glass slides in the same manner as the other samples. As determined by CFU counting, this population is composed of 21 ± 2% of the cells from the original culture that survived desiccation under vacuum, but is here considered as the starting point for our experiments. It can be observed that cell survival fell sharply with the first VUV fluences applied, but presented a pronounced tailing effect upon further irradiation. This prolonged tail in the survival curve is probably due to the presence of a small fraction of shaded cells, as could be expected from the SEM images (Fig. 4). Going beyond what can be observed by counting CFU, molecular probes were applied to the cells and analysed by flow cytometry to distinguish the damage mechanisms of the simulation.

Fig. 5. Experimental results from the interplanetary space simulation at the TGM beamline (VUV irradiation at 10−6 Pa). On top, the survival curve for D. radiodurans under VUV determined by plating and CFU counting is presented (N/N 0, ‘N 0’ being vacuum-exposed, non-irradiated samples). In the middle, the graph shows the proportion of cells with membrane damage (stained with PI) exposed to different VUV fluences enumerated by flow cytometry. At the bottom, it is shown the increase in mean fluorescence of the cell populations caused by the oxidized product of DHR, indicating oxidative stress, also measured by flow cytometry.
The PI staining shows that at the 0 J m−2 point (N 0) about one-fifth of the cells already had damaged membranes (Fig. 5). These measured injuries were caused solely by desiccation and the exposition to the ultra-high vacuum, but it is interesting to note that most of the ‘undamaged’ cells were already non-viable at this point (as present above for the 21 ± 2% viability of these non-irradiated controls in comparison to the cultures). Since this initial desiccation treatment can affect both membrane integrity and generate intracellular oxidative stress (Fredrickson et al., Reference Fredrickson, Li, Gaidamakova, Matrosova, Zhai, Sulloway, Scholten, Brown, Balkwill and Daly2008), it is important to notice that all further analyses were made from this experimental background damage level to the cells.
At the first 200 J m−2 of VUV radiation, survival quickly fell over 90% in relation to this initial population (N 0), while PI staining increased less than 10% (Fig. 5). This is possibly the most informative data point form these experiments, since it is less affected by the tailing effect. Increasing VUV fluences lead to membrane injuries in up to 50–60% of the cells, despite causing further inactivation by nearly 100-times. These results suggest that most cell killing was caused by factors independent of membrane damage, which were also evidenced to happen by the intracellular oxidative stress measurements described next.
Fluorescence from DHR stained cells, indicating oxidative stress, increased over four-fold with the VUV irradiation of the initial population (Fig. 5). A plateau was seemingly reached after about the 1200 J m−2 point, possibly indicating the maximum amount of reactive oxygen species generated for the exposed cells (whose absolute majority was already inactivated). The difference between the 0 and 200 J m−2 points, despite seeming small, could already be a signal of the severe inactivation that the cells suffered. Further oxidation of the probe could have proceeded in already dead cells. Nevertheless, it remains probable that additional direct damages not grasped by the techniques applied in this study also occurred.
Discussion
It is known that the main mechanism of action of ultraviolet in the >200 nm range, when studied alone, is causing the formation of photoproducts on DNA and oxidatively damaging proteins (Pogoda de la Vega et al., Reference Pogoda de la Vega, Rettberg, Douki, Cadet and Horneck2005; Krisko and Radman, Reference Krisko and Radman2010; Santos et al., Reference Santos, Oliveira, Baptista, Henriques, Gomes, Almeida, Correia and Cunha2013). Still, UV has already been shown to be also capable of injuring the cell membrane of D. radiodurans, but only after exposure to sufficiently high fluences (Nellen et al., Reference Nellen, Rettberg, Horneck, Harris and Ouwehand2003). A possible mechanism for that is the oxidation of membrane lipids, which can be induced by UV (Santos et al., Reference Santos, Oliveira, Baptista, Henriques, Gomes, Almeida, Correia and Cunha2013), leading to the formation of pores and causing the permeabilization of the cells (Pashkovskaya et al., Reference Pashkovskaya, Kotova, Zorlu, Dumoulin, Ahsen, Agapov and Antonenko2010).
Membrane integrity is essential for numerous cellular functions and, fundamentally, the very survival of the cell (Baatout et al., Reference Baatout, De Boever and Mergeay2005). Thus, damage in this structure is a strong indicator of a cell being dead and unable to multiply. On the other hand, one important distinction is that dead cells do not necessarily show permeable membranes (Joux and Lebaron, Reference Joux and Lebaron2000), which can be of special interest in analysis of live/dead sorting based on PI staining as it is commonly used. For example, Fiksdal and Tryland (Reference Fiksdal and Tryland1999) showed that E. coli populations irradiated with UV-C (254 nm) lost viability much faster than their membrane integrity was compromised, while Berney et al. (Reference Berney, Weilenmann and Egli2006) demonstrated the same effect for these cells under UV-A. Similar to these observations, at the VUV synchrotron beamline experiments, it was noticed that the membrane damage increased at a much lower rate than the cellular inactivation (Fig. 5). Despite the particular mechanism through which VUV affects cells still not being well understood, this was unexpected, as explained below.
The VUV range of radiation is highly interacting and penetrates very little in biological matter. This has led to the suggestion that its damage mechanism is localized primarily at the cell membrane and surface structures (Coohill, Reference Coohill1986; Ito, Reference Ito, Sweet and Woodhead1989). D. radiodurans' singular cell envelope is composed of at least five different layers, including a Gram-positive-like robust cell wall and a Gram-negative-like outer membrane, adding up to a thickness of near 150 nm (Slade and Radman, Reference Slade and Radman2011). Its outermost component is a paracrystalline protein surface layer, called the S-layer, which surrounds the cells. The main protein of this structure forms a complex with the carotenoid deinoxanthin and has been studied as the first line of defence of this organism against environmental insults such as UV and desiccation (Farci et al., Reference Farci, Slavov, Tramontano and Piano2016). Additionally, Bauermeister et al. (Reference Bauermeister, Moeller, Reitz, Sommer and Rettberg2011) demonstrated that desiccation (a basic requirement for experiments at ultra-high vacuum) increases the resistance of D. radiodurans to ionizing and ultraviolet radiation. These factors could be expected to confer some resistance to these cells to the VUV, but nevertheless they perished rapidly under the simulation. The higher wavelength portion of the TGM beamline spectrum could be partially responsible for injuries beyond the cells' membranes, despite being present at lower intensities (Paulino-Lima et al., Reference Paulino-Lima, Pilling, Janot-Pacheco, de Brito, Barbosa, Leitao and Lage2010; Abrevaya et al., Reference Abrevaya, Paulino-Lima, Galante, Rodrigues, Mauas, Corton and Lage2011). Ito (Reference Ito, Sweet and Woodhead1989) reviewed experiments demonstrating for different biological systems that the effects of wavelengths above 160–170 nm shift from being mainly superficial and start to involve prominently DNA damage, which appears to be related to the increased penetrance of this longer-wavelength radiation.
The survival curve for D. radiodurans exposed to VUV under ultra-high vacuum shows a substantial tailing effect, which indicates that a small proportion of cells was clustered, shaded or otherwise partially protected from the radiation. The UV protection from shading materials has already been studied in Mars simulations (Diaz and Schulze-Makuch, Reference Diaz and Schulze-Makuch2006; Osman et al., Reference Osman, Peeters, La Duc, Mancinelli, Ehrenfreund and Venkateswaran2008; Smith et al., Reference Smith, Schuerger, Davidson, Pacala, Bakermans and Onstott2009) and also for the VUV (Paulino-Lima et al., Reference Paulino-Lima, Pilling, Janot-Pacheco, de Brito, Barbosa, Leitao and Lage2010). In fact, the SEM images of the samples (Fig. 4) show the presence of substantial cell clumping and precipitated salt crystals that may have conferred some protection to the persisting fraction of survivors recovered at the tail end of the survival curve. This interesting data presents some contrast to the findings that the beamline radiation can exert influence to further depths beyond the immediate surface of microorganisms: single exposed cells may perish, but even microscopic-level shading can raise their survivability significantly.
Adding up to the findings that most cells were inactivated in a membrane damage-independent manner, oxidative stress within the microorganisms was also measured. Krisko and Radman (Reference Krisko and Radman2010) demonstrated that the production of reactive oxygen species (as measured by DHR) is correlated with the inactivation of E. coli and D. radiodurans exposed to gamma radiation and UV-C. This supports the relevance of this probe as a marker of a significant type of damage to microbial cells. At the interplanetary space simulation performed in the synchrotron beamline, the DHR fluorescence indicated intracellular oxidative stress induced by the VUV radiation increasing steadily with irradiation with the first fluences, and then rising up to over four-fold the initial values (Fig. 5). It is worth noting that under ultra-high vacuum the amount of remaining intracellular water is reduced, diminishing its potential to generate free radicals upon irradiation. Apart from possible direct effects of VUV in inner cell components, despite its reduced potential to propagate through matter, the oxidation of DHR can also be attributed to endogenous reactive oxygen species generated upon rehydration of surface damaged cells ineffectively trying to restart their metabolism. The respiratory chain of aerobic organisms is known to generate these types of compounds (Cabiscol et al., Reference Cabiscol, Tamarit and Ros2010), which might be exacerbated in cells injured from UV, desiccation, etc.
Conclusions
An ultra-high vacuum chamber mounted on a VUV synchrotron beamline was used to assay the effects of a simulated interplanetary space environment over a model extremotolerant bacterium, D. radiodurans. The highly interacting VUV was expected to damage mainly the cells' membranes, though this was observed to be a minor contributor to inactivation. Intracellular effects of this radiation were further evidenced by the measurement of reactive oxygen species generated within cells. In this manner, the presented results support that less superficial types of damage should be involved under these replicated extraterrestrial conditions. An additional interesting result is that despite VUV seemingly not being limited by the outermost layers of microorganisms, microscopic protection conferred by cell clumping and precipitated salts suffices to prolong the survival of cells to very high fluences of this radiation. Finally, from a methodological standpoint, this study contributes to the exploration of different strategies to evaluate the response of living systems under simulations of astrobiological importance.
Acknowledgements
This work was sponsored by FAPESP (Project 2012/18936-0), CAPES, CNPq (Project 424367/2016-5), CNPq – Proantar, USP, through the Brazilian Research Unity in Astrobiology – NAP/Astrobio and the Serrapilheira Institute (Project G-1709-20205). The authors acknowledge the Brazilian Synchrotron Light Laboratory – LNLS, for the use of the Toroidal Grating Monochromator beamline (TGM) under the proposal TGM – 16126, the Brazilian Biosciences National Laboratory – LNBio for the use of facilities, Maria Eugenia Ribeiro de Camargo for operating the flow cytometer, the Brazilian Nanotechnology National Laboratory (LNNano – CNPEM, Campinas, Brazil) for the use of the FEI Quanta 650 FEG scanning electron microscope and the staff of the Brazilian Astrobiology Laboratory – AstroLab where most of the experiments were conducted.
Conflict of interest
No competing financial interests exist.








