Introduction
Optimal foraging theory predicts that organisms seek to maximize net energetic yield in minimum foraging time (Grundel Reference Grundel1992, Oster & Wilson Reference Oster and Wilson1978, Stephens & Krebs Reference Stephens and Krebs1986), and that this drives animal behaviour in terms of food selection and decisions about where, when and for how long foraging occurs (Pyke et al. Reference Pyke, Pulliam and Charnov1977, Schoener Reference Schoener1971). According to optimal foraging theory (MacArthur & Pianka Reference MacArthur and Pianka1966), central-place foragers such as social insects optimize net energy gain by balancing trade-offs relating to colony location in a way that maximizes fitness (Covich Reference Covich1976, Orians & Pearson Reference Orians, Pearson, Horn, Mitchell and Stairs1979, Schoener Reference Schoener1979). Several studies have demonstrated that the foraging of social insects can be optimized by the location of nests close to key food resources (Grundel et al. Reference Grundel, Jean, Frohnapple, Glowacki, Scott and Noel2010, Kacelnik et al. Reference Kacelnik, Houston and Schmid-Hempel1986, Murray Reference Murray1938, Potts et al. Reference Potts, Vulliamy, Dafni, Ne’eman, O’Toole, Roberts and Willmer2003). However, there have been few empirical studies addressing how the distribution of food resources influences spatial patterns of social insect colonies.
Ants are ecologically dominant social insects in most terrestrial ecosystems, living in highly organized colonies where foragers retrieve food items to their nests, where they are stored, eaten or fed to offspring (Hölldobler & Wilson Reference Hölldobler and Wilson1990, Traniello Reference Traniello1989). Plants provide many resources for ants as part of mutualistic interactions involving defence against herbivores (Bennett & Breed Reference Bennett and Breed1985, Bequaert Reference Bequaert1922). Such resources can include nesting sites in the form of hollow thorns, stipules, leaf pouches, and chambers within epiphytic tubers (Janzen Reference Janzen1966, Rico-Gray & Oliveira Reference Rico-Gray and Oliveira2007). However, most mutualistic interactions between plants and ants involve ant species that make their own nests, and therefore make their own decisions about where to locate their nests in relation to plant-based food resources such as leaves, nectar, seeds, honeydew, or insects that live on vegetation (Holway & Case Reference Holway and Case2000, Kay Reference Kay2002, Wagner & Fleur Nicklen Reference Wagner and Fleur Nicklen2010).
Extrafloral nectar (EFN) is a carbohydrate-rich food resource produced by at least 3941 plant species (Weber & Keeler Reference Weber and Keeler2013, Zhang et al. Reference Zhang, Zhang and Keming2015) for attracting ants, which helps protect plants from herbivores (Heil Reference Heil2011, Rico-Gray & Oliveira Reference Rico-Gray and Oliveira2007). Many specialist nectar-feeding ants have specialized digestive systems designed to exploit liquid carbohydrates, allowing intensified exploitation of such resources, and thus generating high fidelity with their host plants (Byk & Del-Claro Reference Byk and Del-Claro2011, Davidson Reference Davidson1997). Many studies have shown that the availability of EFN strongly influences arboreal ants, promoting ant foraging activity and diversity on trees (Blüthgen & Fiedler Reference Blüthgen and Fiedler2004, Davidson et al. Reference Davidson, Cook and Snelling2003, Davidson Reference Davidson1997, Koptur Reference Koptur and Bernays1992), and increasing colony survivorship, growth and reproduction (Byk & Del-Claro Reference Byk and Del-Claro2011). EFN is also exploited by ground-nesting ants, but its role in structuring ground-nesting ant communities has received little attention. Previous studies suggest that EFN production may encourage ants to build their nests near plants (Holway & Case Reference Holway and Case2000, Van Wilgenburg & Elgar Reference Van Wilgenburg and Elgar2007, Wagner & Fleur Nicklen Reference Wagner and Fleur Nicklen2010), but the community-wide influence of EFN on the spatial structure of ground-nesting ants remains to be examined.
In this study, we investigate the relationships between the distributions of EFN-producing plants and the nests of epigaeic ants occurring in caatinga dry forest of north-eastern Brazil. First, we hypothesize that the availability of EFN-producing plants plays an important role in structuring the spatial distribution of nests of ant species that are heavily dependent on nectar. We predict that the nests of such species are closer to trees producing EFN than are the nests of other ant species. Second, given that both disturbance and decreasing rainfall can negatively impact both populations of EFN-producing plants and nectar production (Heil Reference Heil2011, Leal et al. Reference Leal, Andersen and Leal2015, Pacini et al. Reference Pacini, Nepi and Vesprini2003), we hypothesize greater effects on the density and distribution of nests of heavily nectar-dependent ant species compared with other ants. Compared with other species, for ant species that are heavily dependent on nectar we predict a greater reduction in nest density with increasing CAD and decreasing rainfall. We also predict that the nests of ant species that are heavily dependent on nectar will be located even closer to EFN-producing plants with increasing CAD and decreasing rainfall, because nectar becomes an increasingly limited resource.
Materials and methods
Study area
The study was carried out in Catimbau National Park (8º24′00′′–8º36′35′′S; 37º09′30′′–37º14′40′′W), in the state of Pernambuco, north-eastern Brazil, located in caatinga dry forest (Figure 1). Caatinga is the world’s most species-rich dry forest (Pennington et al. Reference Pennington, Lavin and Oliveira-Filho2009, Silva et al. Reference Silva, Leal and Tabarelli2017), and a large proportion of its woody plant species produce EFN (Leal et al. Reference Leal, Andersen and Leal2015, Melo et al. Reference Melo, Machado and Alves2010). Caatinga is also one of the world’s most highly populated and threatened semi-arid biomes, sustaining 28 million people who are highly dependent on forest natural resources for their livelihoods (Ribeiro et al. Reference Ribeiro, Arroyo-Rodríguez, Santos, Tabarelli and Leal2015, Silva et al. Reference Silva, Leal and Tabarelli2017) in a typical regime of chronic anthropogenic disturbance (Singh Reference Singh1998). Moreover, caatinga is highly threatened by climate change, with climate models forecasting a 22% reduction in rainfall and a 3–6°C increase in temperatures by 2100 (Magrin et al. Reference Magrin, Marengo, Boulanger, Buckeridge, Castellanos, Poveda, Vicuña and Barros2014).
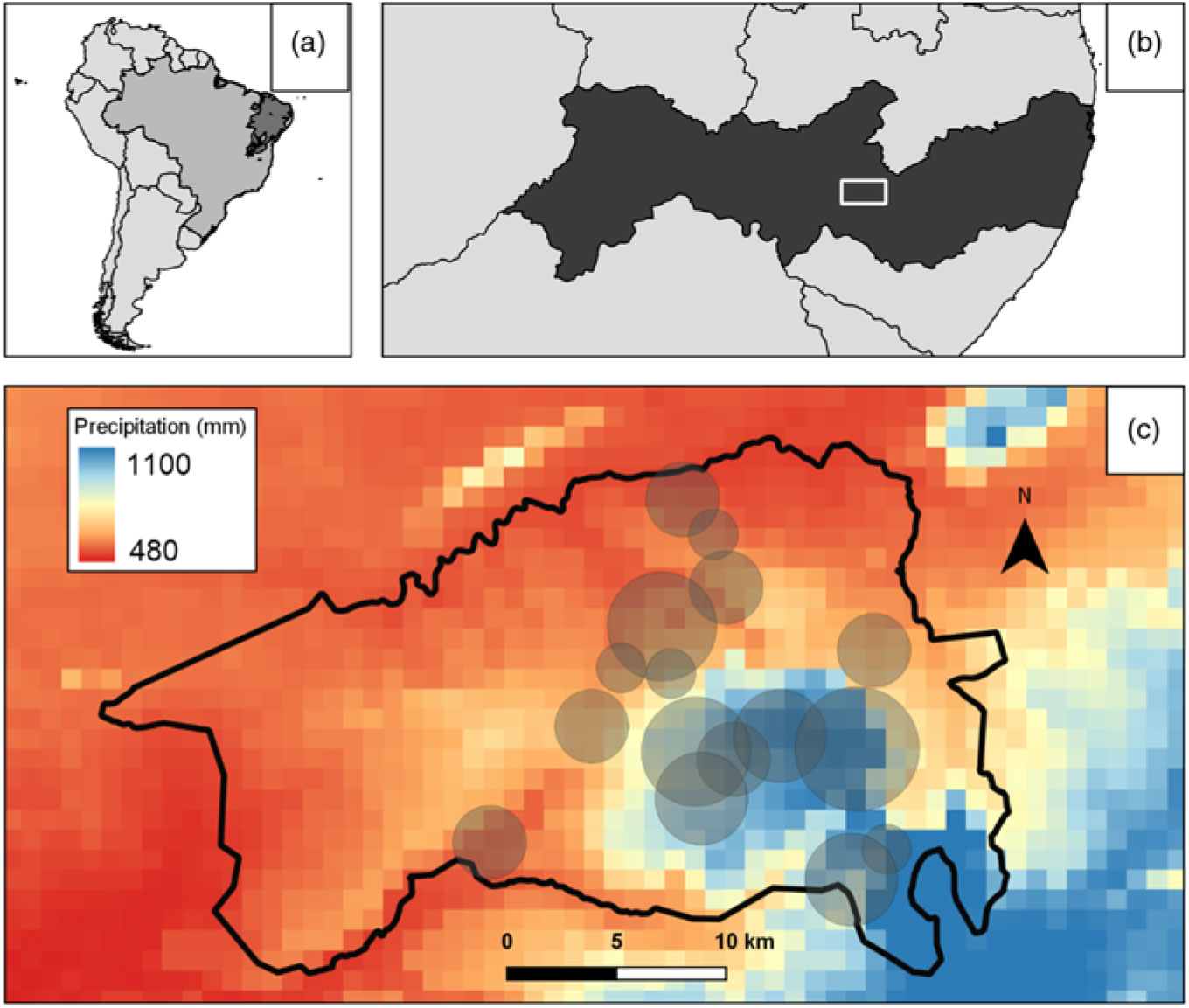
Figure 1. Location of study plots in Catimbau National Park (dark grey) in South America (a), location of Catimbau National Park (white rectangle) in Pernambuco state, Brazil (b), limits of Catimbau National Park (in black) with the 20 study plots represented by grey circles (increasing circle size represents higher levels of disturbance) (c).
The climate in Catimbau National Park is semi-arid, with annual temperature averaging 23°C, and mean annual rainfall varying from 480 to 1100 mm y−1, concentrated between March and July (Sociedade Nordestina de Ecologia 2002). Approximately 70% of its 607 km2 is covered by quartzite sandy soils, but planosols and lithosols are also present (15% each one; Siqueira et al. Reference Siqueira, Ribeiro-Neto, Tabarelli, Andersen, Wirth and Leal2017). The vegetation is composed of shrubs and small trees up to 7 m in height, dominated by the families Leguminosae, Euphorbiaceae, Boraginaceae and Burseraceae (Rito et al. Reference Rito, Arroyo-Rodríguez, Queiroz, Leal and Tabarelli2017). Catimbau National Park is one of the most important conservation areas within the caatinga dry forest (Silva et al. Reference Silva, Tabarelli, Fonseca and Lins2004). However, the Park was only recently established (in 2002) and its inhabitants still remain; therefore, it continues to be subject to a regime of chronic anthropogenic disturbance, including livestock grazing, timber harvesting, firewood collection and hunting (Rito et al. Reference Rito, Arroyo-Rodríguez, Queiroz, Leal and Tabarelli2017, Siqueira et al. Reference Siqueira, Ribeiro-Neto, Tabarelli, Andersen, Wirth and Leal2017, Sociedade Nordestina de Ecologia 2002). Catimbau includes a major gradient of decreasing rainfall from 1100 mm in the south-east to 480 mm in the north-west; such bioclimatic gradients provide powerful frameworks for addressing climate change impacts through space-for-time substitution (Blois et al. Reference Blois, Williams, Fitzpatrick, Jackson and Ferrier2013; Caddy-Retalic et al. Reference Caddy-Retalic, Andersen, Aspinwall, Breed, Byrne, Christmas, Dong, Evans, Fordham, Guerin, Hoffmann, Hughes, van Leeuwen, McInerney, Prober, Rossetto, Rymer, Steane, Wardle and Lowe2017).
Based on satellite imagery, soil maps, and data on rainfall and disturbance, we established 20 0.1-ha plots (50 × 20 m) covering a wide range of rainfall and disturbance intensity. All plots were on sandy soil, on flat terrain, and supported old-growth vegetation that had not experienced slash-and-burn agriculture for at least 50 y (Rito et al. Reference Rito, Arroyo-Rodríguez, Queiroz, Leal and Tabarelli2017). Plots were separated by a minimum of 2 km and located within a total area of 21 430 ha (Rito et al. Reference Rito, Arroyo-Rodríguez, Queiroz, Leal and Tabarelli2017).
Characterizing CAD and rainfall gradients
In order to assess the effects of CAD on the density and distribution of epigaeic ant nests, we used a global multi-metric disturbance index previously established for the study plots (Arnan et al. Reference Arnan, Leal, Tabarelli, Andrade, Barros, Câmara, Jamelli, Knoechelmann, Menezes, Menezes, Oliveira, de Paula, Pereira, Rito, Sfair, Siqueira, Souza, Specht, Vieira, Arcoverde and Andersen2018). The index combines three types of source information: (1) indirect landscape measures using satellite imagery in ArcGIS 10.1 software (proximity to the nearest house, and proximity to the nearest road); (2) socio-economic information obtained by interviewing local households (number of people in the nearest village with influence weighted by distance); and (3) direct measures of disturbance in the field (length of goat trails, the density of goat and cattle dung, and extents of live-wood extraction and fire-wood collection). The values of each disturbance metric were first standardized between 0 and 1 to make component metrics of equal importance. We then computed the global multi-metric index ranged from 2 to 58. Full details on this disturbance index are provided in Arnan et al. (Reference Arnan, Leal, Tabarelli, Andrade, Barros, Câmara, Jamelli, Knoechelmann, Menezes, Menezes, Oliveira, de Paula, Pereira, Rito, Sfair, Siqueira, Souza, Specht, Vieira, Arcoverde and Andersen2018).
We obtained data on mean annual rainfall for each plot from the WorldClim global climate data repository (Hijmans et al. Reference Hijmans, Cameron, Parra, Jones and Jarvis2005) with 1-km resolution using the maptolls package for R v3.1.2. There are six meteorological stations around Catimbau National Park from which rainfall data have been interpolated to estimate values for our plots, which ranged from 510 to 940 mm. Such extreme variation in a small geographic area makes the study area ideal for analysing the ecological effects of rainfall on plant (Rito et al. Reference Rito, Arroyo-Rodríguez, Queiroz, Leal and Tabarelli2017) and ant (Arnan et al. Reference Arnan, Leal, Tabarelli, Andrade, Barros, Câmara, Jamelli, Knoechelmann, Menezes, Menezes, Oliveira, de Paula, Pereira, Rito, Sfair, Siqueira, Souza, Specht, Vieira, Arcoverde and Andersen2018, Leal et al. Reference Leal, Andersen and Leal2015) community attributes and interactions (Câmara et al. Reference Câmara, Leal, Blüthgen, Oliveira, De Queiroz and Arnan2018, Oliveira et al. Reference Oliveira, Andersen, Arnan, Ribeiro-Neto, Arcoverde and Leal2019).
Plant and ant surveys
We used the abundance of EFN-bearing plants as an indirect measure of overall availability of extrafloral nectar. To do so, we identified and counted all adult (> 1.5 m in height and > 3 cm diameter at soil level, following Rodal et al. Reference Rodal, Sampaio, Figueiro and Figueiredo1992) EFN-producing woody plants in each plot between August 2014 and February 2015. Plant species were identified by comparing field-collected samples with samples from the Federal University of Pernambuco herbarium (UFP- Geraldo Mariz Herbaria), where voucher specimens of each species have been deposited. All EFN-producing plants were mapped according to a Cartesian coordinate system within each plot.
We surveyed ant nests in each plot between August 2014 and February 2015 by following foragers attracted to tuna baits returning to their nests. Thirty baits were used in each plot, placed on the soil for 2 h in a 6 × 5 grid with 10-m spacing, located over the 50 × 20-m plots used to sample EFN-producing plants. We chose to use protein- rather than carbohydrate-based baits because these are attractive to a greater range of ant species, including both ants that are and are not heavily dependent on EFN (Andersen Reference Andersen1992). All ant nests were mapped according to the Cartesian coordinate system within each plot. All ants were sorted to morphospecies and identified to genus using Baccaro et al. (Reference Baccaro, Feitosa, Fernández, Fernandes, Izzo, Souza and Solar2015). Voucher specimens of all morphospecies were sent to the Systematics and Ant Biology Laboratory at the Universidade Federal do Parana for species identification. A complete set of voucher specimens is held in the ant collection at the Universidade Federal de Pernambuco. All applicable institutional and/or national guidelines for the care and use of animals were followed.
We categorized ant species into three types according to their dependence on EFN, based on published literature (Davidson Reference Davidson1997; Davidson et al. Reference Davidson, Cook and Snelling2003) and our own expert knowledge: (1) heavy users: ants that are highly dependent on exudates and have special adaptations for a diet consisting primarily of liquid food; (2) occasional users: ants that feed on nectar opportunistically, and do not possess adaptations for it; and (3) non-users: ants that rarely or never feed on nectar.
Data analysis
We constructed a General Linear Mixed Model (GLMM) in order to test whether the nests of ant species that are heavily dependent on EFN are closer to EFN-producing plants than are those of other species, where the distance of each ant nest to the nearest EFN-producing plant was the response variable, and ant feeding type was the fixed factor. Ant species nested within plot was added to the model as random factor. Second, we used Kolmogorov–Smirnov tests to compare the frequency distributions of the nearest distances between ant nests and EFN-producing plants among the three ant feeding types. To test our second prediction that increasing CAD and decreasing rainfall will have greater negative effects on nest density of species that are heavily dependent on nectar than in the other groups, we conducted General Linear Models (GLMs) where the density of nests of all ant species, heavy users, occasional users and non-users were the response variables, and CAD and rainfall were the fixed factors. Finally, to test our third prediction that nests of ant species that are heavily dependent on nectar will be located closer to EFN-producing plants with increasing CAD and decreasing rainfall, we ran a GLMM where the distance of each nest of heavy users to the nearest EFN-producing plant species was the response variable, CAD and rainfall were the fixed factors, and ant species nested within plots was the random factor. For the tests associated to our second and third predictions, we conducted a model comparison approach based on the Akaike’s information criterion with a correction for finite sample sizes (AICc) to select the best-supported models; this approach reduces the problems associated with multiple testing, co-linearity of explanatory variables, and small sample sizes (Burnham & Anderson Reference Burnham and Anderson2002). We compared four models, containing: both CAD and rainfall; only CAD; only rainfall; and only the intercept. The best-supported models were selected based on their AICc weights, which reveal the relative likelihood of a given model based on the data and the fit scaled to one; thus, models with a delta (AICc difference) of <2 were selected (Burnham & Anderson, Reference Burnham and Anderson2002). The relevant variables were those that were retained in the best-supported models (except when the best-supported model consisted only of the intercept). Model selection was carried out using the dredge function in the MuMIn package in R.
Results
A total of 2243 individuals belonging to 12 genera and 21 species of EFN-producing plants were recorded, with a mean (±SE) density of 1151 ± 623 plants ha−1 and mean plot richness of 5.1 ± 0.5 species. Most of the EFN-producing plant species (75%) and individuals (70%) belonged to Leguminosae. The most common species were Pityrocarpa moniliformis (Leguminosae; 27.8% of total individuals); Poincianella microphylla (Leguminosae; 19.6%) and Croton argyrophylloides (Euphorbiaceae; 18.8%). The density of EFN-bearing plants was not associated with variation in either CAD (GLM: F1,17 = 0.7; P = 0.391) or rainfall (F1,17 = 0.5; P = 0.482).
We recorded 257 ant nests belonging to 33 species (Table 1). The number of nests per plot varied from 5 to 24, corresponding to a density range of 50–240 nests ha−1. The most common ant species were Ectatomma muticum (Ectatomminae; 22.5% of all nests; occurring in 12 plots), Dorymyrmex goldii (Dolichoderinae; 10.8%; 9) and D. thoracicus (7.8%; 5). Heavy users comprised seven species of Dorymyrmex, which collectively represented 93% of all heavy-user nests, along with Camponotus crassus. Heavy users, occasional users and non-users accounted for 37.3%, 41.2% and 21.5% respectively of all ant nests. Ant nest density of any feeding category was not related to the density of EFN-producing plants.
Table 1. List of ground-nesting ant species per subfamily showing feeding type (H = heavy user; O = occasional user; N = non-user) in relation to extrafloral nectar, total number of nests, and number of plots in which it occurred in Catimbau National Park, Pernambuco State, Brazil.
Mean nest distance from the nearest EFN-producing plant varied significantly among ant feeding types (F2,83 = 23.4; P <0.001), with heavy-users (mean distance 1.1 ± 0.51 m) nesting closer to EFN-producing plants than did occasional users (1.7 m; ±1.22), which in turn nested closer to EFN-producing plants than did non-users (2.3 m; ±2.03; Figure 2). Most nests of heavy users were located within 1 m of the nearest EFN-bearing plant, and none was located >4 m distant (D = 0.32; P <0.001). In contrast, most nests of other feeding types were located >1.5 and some >6 m distant (D = 0.50; P <0.001) (Figure 3).

Figure 2. Boxplots showing the nearest distance between ant nests and EFN-bearing plants for the three ant feeding types according to EFN dependence (heavy-users, occasional users, and non-users) in Catimbau National Park, north-eastern Brazil. Different letters indicate significant differences (P < 0.05) according to post hoc contrasts.
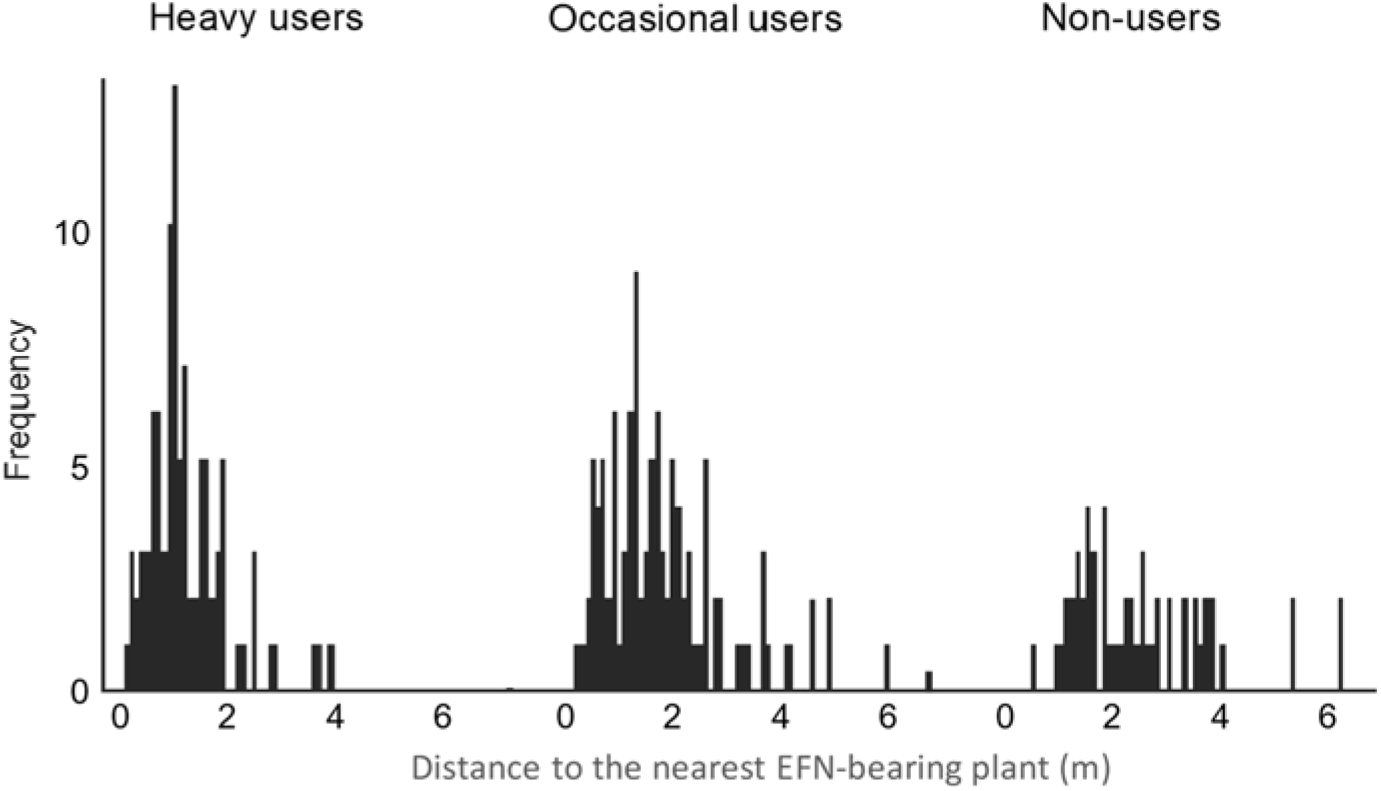
Figure 3. Distribution of the nearest distances between ant nests and EFN-producing plants for the three ant feeding types according to EFN dependence (heavy-users, occasional users and non-users) in Catimbau National Park, north-eastern Brazil.
The best-supported models always consisted only of the intercept, except for the density of heavy users, which included rainfall (Table 2). Thus, there was no relationship between CAD and the density of total ant nests or of those of any feeding type. The density of nests of heavy users declined with decreasing rainfall (Figure 4), but there was no relationship for either occasional users or non-users. Similarly, neither CAD nor rainfall were related to the proximity of heavy-user nests to EFN-producing plants (Table 2).
Table 2. Statistical outputs from the model comparison approach conducted to test the effects of chronic anthropogenic disturbance (CAD) and rainfall on the density of total nests, ant ground nests of heavy-users, occasional users and non-users and on the proximity of heavy users to EFN-bearing plants in Catimbau National Park, north-eastern Brazil. The best-supported models are highlighted by *. Int., Intercept.
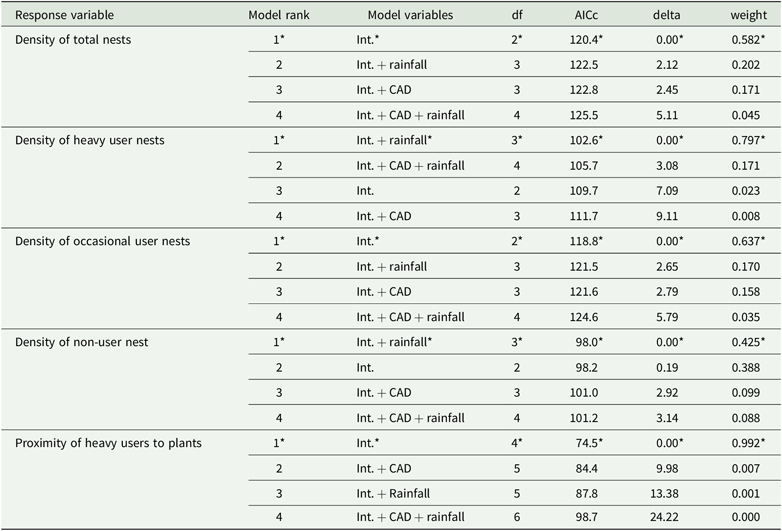
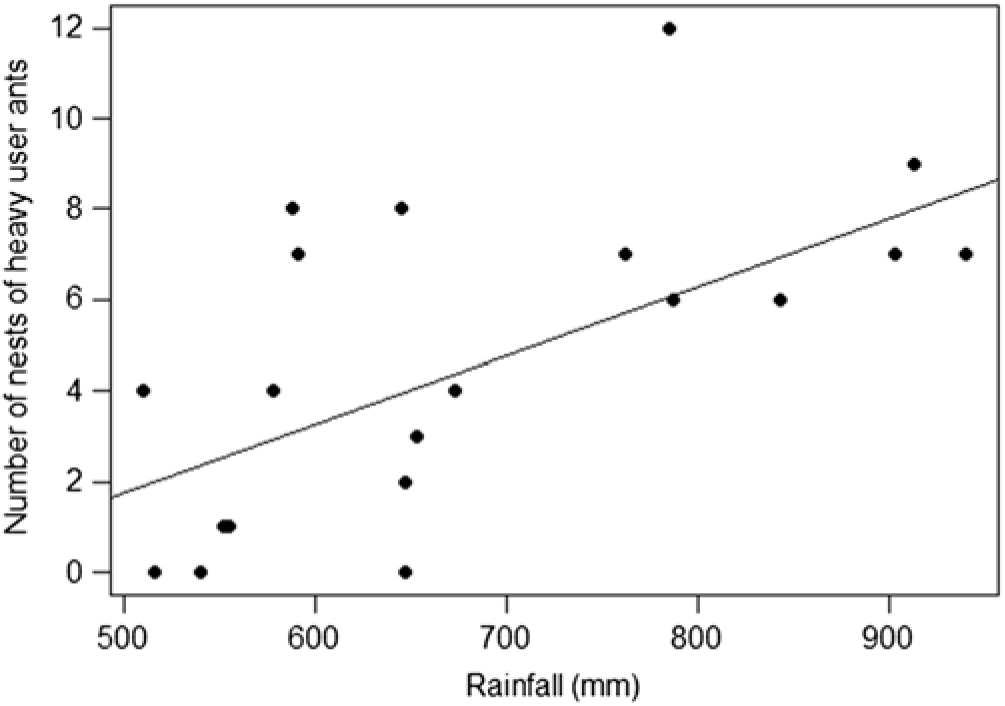
Figure 4. Relationship between mean annual rainfall and the density of nests of EFN heavy-user ants in the 20 study sites at Catimbau National Park, north-east Brazil. The line depicts the linear fit between the two variables.
Discussion
The availability of EFN is an important factor influencing arboreal ant communities, but its effects on ground-nesting ants remains largely unknown. Our study examined the relationship between the locations of EFN-producing plants and ant nests, and whether this varies along gradients of CAD and rainfall, both of which are known to influence EFN production. For the first time, we have shown that EFN-bearing plants are a key driver of the spatial structure of entire ground-nesting ant communities. This occurred across a wide range of disturbance and climatic conditions.
Species of Dorymyrmex and Camponotus were heavily dependent on EFN (heavy users) in our study system. They belong to subfamilies (Dolichoderinae and Formicinae respectively) that are considered to be specialized exudate feeders (Eisner Reference Eisner1957), possessing anatomical traits that allow them to retain and process large volumes of plant exudates (Davidson & Patrell-Kim Reference Davidson, Patrell-Kim and Gibson1996, Davidson et al. Reference Davidson, Cook and Snelling2003, Eisner Reference Eisner1957). Species of Camponotus have a sclerotized proventriculus that can store large volumes of fluids for long periods of time (Eisner Reference Eisner1957). As in other dolichoderine genera such as Iridomyrmex, Froggattella and Turneria, species of Dorymyrmex possess a complex proventriculus that is capable of carrying more liquid than is required by an individual forager (Cook & Davidson Reference Cook and Davidson2006, Eisner Reference Eisner1957).
Nesting in close proximity to a key food resource reduces travel times, which both increases foraging efficiency and reduces exposure to natural enemies (Davidson Reference Davidson1997, McIver Reference McIver1991, Pfeiffer & Linsenmair Reference Pfeiffer and Linsenmair1998). As we predicted, heavy-user ants established their nests closer to EFN-bearing plants than did other ants. Mean nest distance to the nearest EFN-producing plant was 1.2 m for heavy users, compared with about 2 m for other ant species. Most nests of heavy users were located within 1 m of an EFN-producing plant, whereas most nests of other species occurred more than 1.5 m away. We also found a more-nuanced effect of ant feeding type: although nests of occasional users were more distant than those of heavy users, they were closer than those of non-users. Our study therefore provides empirical evidence that EFN drives the nest distributions of ant species according to the extent to which they use nectar resources.
A relationship between nest distribution and nectar resources has been previously suggested for individual ground-nesting ant species. For example, Bennett & Breed (Reference Bennett and Breed1985) found an association between Pentaclethra macroloba (Leguminosae) trees and nests of the giant tropical ant Paraponera clavata in Costa Rica, likely related to the provision of EFN. Similarly, there is an association mediated by EFN between Acacia constricta and Dorymyrmex and Forelius species in the Sonoran Desert (Wagner & Fleur Nicklen Reference Wagner and Fleur Nicklen2010). However, our study is the first to demonstrate a community-wide impact of EFN on the spatial structure of epigaeic ants.
Many heavy-user ant species are behaviourally dominant, relying on large volumes of liquid carbohydrate for powering their rapid locomotory activity and high levels of aggression (Blüthgen & Fiedler Reference Blüthgen and Fiedler2004, Davidson et al. Reference Davidson, Cook and Snelling2003). In our study, this applies to species of Dorymyrmex (Dominant Dolichoderinae sensu Andersen Reference Andersen1995), which represented >90% of all heavy-user nests. These dominant species can exercise competitive control in the vicinity of their nests (Hölldobler & Wilson Reference Hölldobler and Wilson1990), and this is likely to be a factor contributing to other species nesting more distantly from EFN-producing plants. However, given that the density of Dorymyrmex nests (mean of 41.5 ha−1) was only a small fraction of that of EFN-producing plants (1151 plants ha−1) such competitive exclusion could not be the dominant factor driving the spatial structure of occasional-user and non-user ant species. This is supported by non-significant correlations between the distance to the nearest EFN-bearing plant of Dorymyrmex species nests and the distance to the nearest EFN-bearing plant of occasional users (Spearman rho = −0.18; P = 0.450) and non-users (Spearman rho = −0.28; P = 0.235).
Our study also examined the effects of two global drivers of biodiversity decline, CAD and climate change, on nests of ground-foraging ants and their spatial relationships with EFN-producing plants. We predicted that increasing anthropogenic disturbance and decreasing rainfall would have a greater impact on nest densities of ants that are heavily dependent on nectar than on other species, because disturbance and decreasing rainfall can negatively impact both populations of EFN-producing plants and nectar production (Heil Reference Heil2011, Leal et al. Reference Leal, Andersen and Leal2015, Nichol & Hall Reference Nichol and Hall1988, Whitford et al. Reference Whitford, Martinez-Turanzas and Martinez-Meza1995). Nest density did not vary with CAD, but our prediction held for rainfall, where a decline in rainfall was associated with a decrease in the nest density of heavy-users, but not of other ants. The decline in nest density of heavy-users was not due to a decline in the density of EFN-bearing plants (which did not vary with either rainfall or CAD); it can therefore be attributed to a decline in nectar production rates, which is known to occur under conditions of low water availability (Jakobsen & Kristjánsson Reference Jakobsen and Kristjánsson1994, Keasar et al. Reference Keasar, Sadeh and Shmida2008, Murcia Reference Murcia1995) due to stomatal closure induced by water stress (Heil Reference Heil2011, Lange et al. Reference Lange, Dáttilo and Del-Claro2013, Rico-Gray et al. Reference Rico-Gray, Garcia-Franco, Palacios-Rios, Iz-Castelazo, Parra-Tabla and Navarro1998). This is supported by the finding that the size of EFN glands of P. moniliformis decreases with decreasing rainfall (Reis Reference Reis2016). The decline in nest density of heavy-user ants with increasing aridity suggests that EFN-mediated ant protection services for plants also declines with aridity. This has implications for plant protection services under future climates, which are projected to be substantially drier in the region.
Our final prediction was that the nests of ant species that are heavily dependent on nectar will be located even closer to EFN-producing plants with increasing disturbance and decreasing rainfall, because nectar becomes an increasingly limited resource (Heil Reference Heil2011, Leal et al. Reference Leal, Andersen and Leal2015, Pacini et al. Reference Pacini, Nepi and Vesprini2003). The density of heavy-user nests did not vary with CAD, which suggests that nectar production likewise did not vary; in such circumstances our finding that proximity of heavy-user nests to EFN-producing plants did not vary with CAD is to be expected. However, nectar production did appear to decline with decreasing rainfall, but this did not result in heavy-user ants nesting even closer to EFN-bearing plants. One possible explanation is that colony size declines with increasing aridity due to factors unrelated to EFN production, such that even if nectar production declines in an absolute sense, it is not relatively more limiting. Alternatively, rate of nectar production might just not be a relevant factor in the optimization of nest proximity to EFN-bearing plants in our study system.
In conclusion, we have shown that EFN is not just important for arboreal ant communities, but that it is a key driver of the spatial structure of ground-nesting ant communities, especially through the supply of a key food resource for behaviourally dominant ants. This has a direct effect on the location of the nests of these ants in relation to EFN-producing plants, and likely also a competition-mediated indirect effect on the spatial structure of other ant species. These effects occur across a wide range of disturbance and climatic conditions.
Acknowledgements
We are grateful to Leila Brito Gonçalves and the many students who assisted with fieldwork. We also wish to thank the Catimbau National Park landowners for giving us permission to work on their properties. CHFS acknowledges the Commonwealth Scientific and Industrial Research Organization (CSIRO, Darwin) for support and cooperation. CNPq receives thanks from XA for the postdoctoral grant (PDS-167533/2013-4 and PDS-165623/2015-2), and from IRL for her productivity grants (Produtividade 305611/2014-3).
Financial support
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq processes PELD 403770/2012-2 and Edital Universal 470480/2013-0), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES process PVE 88881.030482/2013-01), Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE processes APQ-0738-2.05/12, APQ- 0138-2.05/14) and Rufford Small Grants Foundation (RSG 17372-1).








