INTRODUCTION
Lianas, or woody climbers, are frequent in tropical forests (Ghazoul & Sheil Reference GHAZOUL and SHEIL2010). Lianas climb trees into the forest canopy, where they may extend from tree-to-tree for tens of metres (Ewers et al. Reference EWERS, ROSELL, OLSON and Hacke2015, Putz Reference PUTZ1984). The abundance, biomass and species richness of lianas, the variety of their climbing mechanisms, and the convergent evolution of the liana life form in numerous plant families (e.g. Fabaceae, Sapindaceae, Bignoniaceae) testify to the adaptive success of this climbing strategy (Darwin Reference DARWIN1865, Gentry Reference GENTRY, Putz and Mooney1991, Gianolo Reference GIANOLO, Schnitzer, Bongers, Burnham and Putz2016, Putz Reference PUTZ1983). Lianas compete with trees for light, water and nutrients (Campbell & Newbery Reference CAMPBELL and NEWBERY1993, DeWalt et al. Reference DEWALT, SCHNITZER and DENSLOW2000), and the weight of lianas can cause tree damage, especially in strong winds. Thus lianas can have negative effects on tree recruitment, survival, growth and reproduction (Schnitzer & Carson Reference SCHNITZER and CARSON2010, Schnitzer et al. Reference SCHNITZER, DALLING and CARSON2000, Tobin et al. Reference TOBIN, WRIGHT, MANGAN and SCHNITZER2012, Van der Heijden et al. Reference VAN DER HEIJDEN, PHILLIPS, SCHNITZER, Schnitzer, Bongers, Burnham and Putz2015, Visser et al. In press). It is therefore potentially significant for tropical forests that liana abundance and biomass are increasing in the Neotropics (Phillips et al. Reference PHILLIPS, MARTÍNEZ, ARROYO, BAKER, KILLEEN, LEWIS, MALHI, MENDOZA, NEILL and VARGAS2002, Schnitzer & Bongers Reference SCHNITZER and BONGERS2011, Schnitzer et al. Reference SCHNITZER, MANGAN, HUBBELL, Schnitzer, Bongers, Burnham and Putz2015, Wright et al. Reference WRIGHT, CALDERÓN, HERNÁNDEZ and PATON2004). This increase may reduce tree density and diversity (Chave et al. Reference CHAVE, OLIVIER, BONGERS, CHÂTELET, FORGET, VAN DER MEER, NORDEN, RIÉRA and CHARLES-DOMINIQUE2008, Ingwell et al. Reference INGWELL, WRIGHT, BECKLUND, HUBBELL and SCHNITZER2010, Schnitzer Reference SCHNITZER, Schnitzer, Bongers, Burnham and Putz2015), as well as the capacity of Neotropical forests to store carbon (Schnitzer & Bongers Reference SCHNITZER and BONGERS2011, Schnitzer et al. Reference SCHNITZER, VAN DER HEIJDEN, MASCARO and CARSON2014). Additionally, at the landscape level, liana stem density has been found to increase significantly in highly deforested landscapes in comparison to landscapes with intermediate or low deforestation levels (Arroyo-Rodríguez & Toledo-Aceves Reference ARROYO‐RODRÍGUEZ and TOLEDO‐ACEVES2009). Recording liana population and community dynamics is important for understanding the factors that control liana abundance and their influence on tree demographics.
Lianas proliferate in the high light and climbing opportunities on forest edges, and thus lianas increase following natural forest disturbance and human disturbances such as logging and creation of edges due to forest fragmentation (DeWalt et al. Reference DEWALT, SCHNITZER and DENSLOW2000, Laurance et al. Reference LAURANCE, PÉREZ-SALICRUP, DELAMÔNICA, FEARNSIDE, D'ANGELO, JEROZOLINSKI, POHL and LOVEJOY2001, Putz et al. Reference PUTZ, LEE and GOH1984, Schnitzer & Bongers Reference SCHNITZER and BONGERS2011, Wright et al. Reference WRIGHT, CALDERÓN, HERNÁNDEZ and PATON2004). On Barro Colorado Island, Panama, spatial patterns in liana distributions reflect past disturbance history; areas of greater past disturbance have more spatially aggregated distributions of lianas (Ledo & Schnitzer Reference LEDO and SCHNITZER2014). Liana abundance is usually greatest in 50-year-old or older forests, but declines with increasing stand age (Barry et al. Reference BARRY, SCHNITZER, BREUGEL and HALL2015, Letcher & Chazdon Reference LETCHER and CHAZDON2009, Pérez-Salicrup et al. Reference PÉREZ-SALICRUP, SORK and PUTZ2001). As with trees, while liana density declines, liana basal area and biomass increases with stand age, when surviving lianas grow large and thus indicate old-growth tropical forests (Budowski Reference BUDOWSKI1965, Reference BUDOWSKI1970). However, this connection between lianas and disturbance is complicated by spatially congruent disturbances of different intensity, frequency and spatial extent, such as hurricane disturbance superimposed on forest patches of different past land-use history (Hogan et al. Reference HOGAN, ZIMMERMAN, THOMPSON, NYTCH and URIARTE2016, Uriarte et al. Reference URIARTE, CANHAM, THOMPSON, ZIMMERMAN, MURPHY, SABAT, FETCHER and HAINES2009).
Here we describe how forest disturbance by two hurricanes and historical land-use legacies have affected liana community structure and composition in a Puerto Rican forest in 2001 and 2015. We hypothesized that: (1) Liana abundance and basal area will increase after the forest canopy incurred hurricane damage if the lianas were able to rapidly recolonize the forest canopy as the canopy regrew. (2) The intensity of previous land-use history will impact liana dynamics as a consequence of the differences in tree species occupying areas of different land-use history. (3) Lianas will show an increase in number, biomass, and fruit and flow production, in common with other Neotropical forests, but that changes in liana abundances, biomass and reproduction will be greater in areas exhibiting more successional recovery of the forest from hurricane disturbance, that is areas of more-secondary forest with greater-intensity of past land use.
STUDY SITE
We conducted this study in the El Verde Research Area of El Yunque National Forest in north-eastern Puerto Rico. El Verde is in the subtropical wet Holdridge life zone (Ewel & Whitmore Reference EWEL and WHITMORE1973). Since 1975, it has received an average of 3685 mm y−1 of rainfall; altitudes range from 332 to 427 m asl; topography is dissected and steep in places; and soils are volcanically derived, deeply weathered Oxisols and Ultisols (Soil Survey Staff, 1995). Canopy trees reach 30 m in height, but the main canopy, after it has recovered for a few decades after hurricane effects, is about 20 m high and markedly smooth (Brokaw et al. Reference BROKAW, FRAVER, GREAR, THOMPSON, ZIMMERMAN, WAIDE, EVERHAM, HUBBELL, FOSTER, Losos and Leigh2004). There are about 90 species ha−1 of tree ≥ 10 cm dbh (diameter at 1.3 m above the ground; Thompson et al. Reference THOMPSON, BROKAW, ZIMMERMAN, WAIDE, EVERHAM, SCHAEFER, Losos and Leigh2004).
Hurricane Hugo struck the El Yunque forest in 1989, felling trees, stripping standing trees of leaves and limbs, and exposing most of the area under forest canopy to high light (Brokaw & Grear Reference BROKAW and GREAR1991, Zimmerman et al. Reference ZIMMERMAN, EVERHAM, WAIDE, LODGE, TAYLOR and BROKAW1994). In 1990 a 16-ha permanent forest dynamics plot was established (Luquillo Forest Dynamics Plot: LFDP; with south-west corner at 18°20′N, 62°49′W; measuring 320 × 500 m, comprising 400 contiguous 20 × 20-m quadrats, Thompson et al. Reference THOMPSON, BROKAW, ZIMMERMAN, WAIDE, EVERHAM, LODGE, TAYLOR, GARCÍA-MONTIEL and FLUET2002, Reference THOMPSON, BROKAW, ZIMMERMAN, WAIDE, EVERHAM, SCHAEFER, Losos and Leigh2004; for a map of the LFDP see Figure 1 in Hogan et al. Reference HOGAN, ZIMMERMAN, THOMPSON, NYTCH and URIARTE2016). Twenty-five per cent of individuals in the community of the most-common 26 species in the LFDP incurred canopy damage after Hurricane Hugo, resulting in 9.1% stem mortality (Zimmerman et al. Reference ZIMMERMAN, EVERHAM, WAIDE, LODGE, TAYLOR and BROKAW1994). The forest canopy had largely recovered after Hurricane Hugo (Brokaw et al. Reference BROKAW, FRAVER, GREAR, THOMPSON, ZIMMERMAN, WAIDE, EVERHAM, HUBBELL, FOSTER, Losos and Leigh2004) when Hurricane Georges struck the plot in 1998, but Georges had less effect than Hugo because there were fewer large and old trees to damage due to the damage by Hurricane Hugo 9 y earlier (Scatena et al. Reference SCATENA, BLANCO, BEARD, WAIDE, LUGO, BROKAW, SILVER, HAINES, ZIMMERMAN, Brokaw, Crowl, Lugo, McDowell, Scatena, Waide and Willig2012). Canham et al. (Reference CANHAM, THOMPSON, ZIMMERMAN and URIARTE2010) reported that only 25% of trees in the LFDP that were assessed for damage following both hurricanes incurred some damage in Hurricane Georges, with only 10% being completely damaged. Similarly, trees damaged by Hurricane Hugo in an area of forest adjacent to the LFDP that had mortality rates of 5.2% year−1 for 2 years following Hurricane Hugo, were least likely to incur damage by Hurricane Georges (Ostertag et al. Reference OSTERTAG, SILVER and LUGO2005). In addition, the storm trajectories of the two hurricanes was also different with the LFDP area being more protected by the El Yunque mountain peaks during Hurricane Georges.
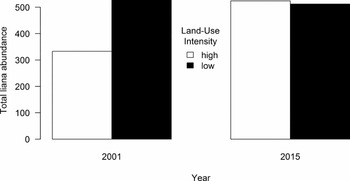
Figure 1. Total recorded liana abundances for the ten 20 × 20-m quadrats (0.4 ha) assessed in the two censuses (2001 and 2015) of the liana community in the Luquillo Forest Dynamics Plot, Puerto Rico. Abundances for the low-intensity land-use portion of the LFDP are shown in white, while the higher-intensity land-use portion of the LFDP is shown in black.
In addition to hurricane disturbance the LFDP also has a history of human land use. Aerial photographs from 1936, historical documents and a timber survey in 1934, show that northern parts of what is now the LFDP had areas that had been clear-cut for wood or planted for coffee and annual crops before 1934 (Hogan et al. Reference HOGAN, ZIMMERMAN, THOMPSON, NYTCH and URIARTE2016, Thompson et al. Reference THOMPSON, BROKAW, ZIMMERMAN, WAIDE, EVERHAM, LODGE, TAYLOR, GARCÍA-MONTIEL and FLUET2002, Weaver Reference WEAVER2012), leaving marginal forest cover, ranging from 0–80% canopy cover (Foster et al. Reference FOSTER, FLUET and BOOSE1999). We term this area the ‘high-intensity land-use’ area of the LFDP. The U.S. Forest Service purchased the forest in the El Verde research area and the surrounding area in 1934, and conducted silvicultural experiments, including a small amount of tree planting in the area, resulting in the establishment of broad-leaved mahoganies (Swietenia macrophylla) and Simarouba amara. The only trees planted, in the 1940s, inside what is now the LFDP was a stand of Calophyllum brasiliense (53 individuals ≥ 10 cm dbh survived to be recorded in the 1990 LFDP census). This area was avoided, by design, for this study. Since 1934, the LFDP including the northern high-intensity land-use area of the LFDP has only been disturbed by ecologists and has regrown to tall forest. The southern part of the plot has always been forested (> 80% forest cover as seen in the 1936 aerial photographs; Foster et al. Reference FOSTER, FLUET and BOOSE1999, Thompson et al. Reference THOMPSON, BROKAW, ZIMMERMAN, WAIDE, EVERHAM, LODGE, TAYLOR, GARCÍA-MONTIEL and FLUET2002) but was lightly, selectively logged and thinned in the 1940s for experiments in stem release for forest management. We term this area the ‘low-intensity land-use’ area of the LFDP.
In 2001, a census of lianas in the LFDP (Rice et al. Reference RICE, BROKAW and THOMPSON2004) showed that liana abundance and species richness were relatively low in this forest when compared with other Neotropical forests, and that there were fewer lianas in the high-intensity land-use area when compared with the low-intensity land-use area. Rice et al. (Reference RICE, BROKAW and THOMPSON2004) hypothesized that recurrent hurricanes have restricted lianas by stripping them from tree canopies, by eliminating tree branches on which some lianas climb, and by increasing the number of trees of species that do not readily support lianas. At the time of the 2001 study, they found some evidence to support this hypothesis, as there was differences in liana abundances between the high-intensity and low-intensity land-use areas.
METHODS
In 2001, 12 y after Hurricane Hugo and 3 y after Georges, we censused lianas in high-intensity and low-intensity land-use areas of the LFDP (Rice et al. Reference RICE, BROKAW and THOMPSON2004). We randomly selected ten 20 × 20-m, non-contiguous quadrats in each land-use area, for a total of 20 quadrats. When censusing lianas it is difficult to determine their rooting points and to trace their routes through the forest canopy. Therefore, we visualized vertical walls extending up from the 20 × 20-m quadrat boundaries, and regardless of rooting point or climbing route, we counted and measured all liana stems that were within that area and ≥ 1.0 cm diameter at 1.3 m height (dbh). It is difficult to distinguish liana individuals; therefore, we counted stems, not individuals and identified lianas to species, when possible. The liana stems were not permanently marked. All trees are identified, mapped, and their diameter is routinely measured as part of the LFDP tree censuses (carried out in 1990,1995, 2000, 2005, 2011 and most recently in 2016), so we knew the location and the diameter of all trees ≥ 10 cm dbh in the quadrats. We did not count hemi-epiphytes, which germinate on trees and send roots to the ground, or germinate in the soil, grow up and eventually lose rooting connection with the ground, unless at the time of the census they still had a stem at 1.3 m from the ground.
We also characterized percentage of tree occupancy of lianas by tree species and the canopy coverage of lianas. Tree occupancy is defined as the percentage of trees (>10cm dbh) hosting lianas. Liana canopy coverage was quantified by estimating classes of: 0, 1–25%, 26–50%, 51–75% or 76–100% of each tree crown inhabited by lianas, as visible to the naked eye or with binoculars. In 2015, we carried out a second liana census of these same 20 × 20-m quadrats using the same protocols as in 2001. Using these data, we calculated changes in tree and liana stem abundance growth, mortality survival and recruitment.
Total liana abundance was compared between censuses using Analysis of Variance (ANOVA), in a basic repeated-measures design accounting for time, where the within-group comparison was collection of quadrats in two areas of differing past land-use intensity. To validate possible differences detected using ANOVA due to pseudoreplication as a result of groups of sampled quadrats lying within land-use intensity areas of the LFDP, liana abundance was modelled using a generalized-linear mixed-model (glmm) with a Poisson probability mass function and log link function, as is the convention for count data (Zurr et al. Reference ZURR, IENO, WALKER, SAVELIEV and SMITH2009). The most parsimonious model was selected using the Akaike Information Criterion (AIC) from all possible combinations of the interaction of land-use intensity and year as fixed effects, and species and quadrats as random effects. The repeated-measures ANOVA was done in the ‘ez’ package and the glmm was fitted using the ‘lme4’ package (Bates et al. Reference BATES, MÄCHLER, BOLKER and WALKER2015) in R 3.2.4. For both censuses of the liana community and for each species we calculated liana biomass using the equation in Schnitzer et al. (Reference SCHNITZER, DEWALT and CHAVE2006): AGB = exp [− 1.484 + 2.657ln(D)], where AGB is above-ground biomass, and D is liana stem diameter.
To compare reproductive effort of lianas between censuses and between the high-intensity and low-intensity land-use areas of the LFDP we used data from a long-term study of flower and fruit production, based on presence of flowers and counts of fruits and seeds, falling into 120 phenology baskets (60 per land-use area) in the LFDP (Wright et al. Reference WRIGHT, MULLER-LANDAU, CALDERON and HERNANDEZ2005, Zimmerman et al. Reference ZIMMERMAN, WRIGHT, CALDERÓN, PAGAN and PATON2007). Seed and flowers were collected from baskets every 2 weeks, the presence of flowers was noted and the number of seeds, immature and mature fruits counted. Pearson chi-square contingency tests (χ 2) were carried out to test for statistical differences between the abundances of liana seeds and presence of flowers, comparing total abundances for eight species for between 2001 and 2015. The chi-square statistical test was used because of its sensitivity in detecting significant differences between count data with few degrees of freedom. Our hypothesis was that there would be more liana fruits and seeds in 2015 than 2001 as the liana abundance would have increased from 2001 to 2015 as the forest recovered from the hurricane damage and that there would be more liana flower and seed production in the high-intensity land-use area when compared with the low-intensity land-use area of the LFDP, because the historically low-intensity land-use area would have fewer reproducing lianas.
RESULTS
Tree population dynamics
Tree species richness and stem density (stems ≥ 10 cm dbh) in the 20 quadrats both declined from 2001 to 2015, as forest structure responded to the hurricane disturbance (Table 1) and pioneer light-demanding trees and hurricane-damaged trees died and new trees grew into the >10 cm dbh size class. In 2001, species richness of the liana host-tree community was a total of 31 species in the 10 censused 20 × 20-m quadrats in the high-intensity land-use area and 33 species in the quadrats of the low-intensity land-use area, but the dominant species were different. By 2015, the species richness had equalized to 30 species in each land-use-history type of area.
Table 1. Tree species richness, density and basal area for all stems (≥ 1 cm, <10 cm dbh) and large stems (≥ 10 cm dbh) from 2001 and 2015 in the 0.4 ha of the Luquillo Forest Dynamics Plot (LFDP) assessed for lianas. All calculations are based upon the 0.4-ha area of the LFDP used for the liana census.

Forest structural changes illustrate the hurricane-disturbance and recovery-dynamics, especially in the small diameter stems (1 cm ≤ dbh < 10 cm), which were nearly double in the 0.4 ha of the high-intensity land-use area compared with the low-intensity land-use area of the plot in 2001. Fourteen years later in 2015, stem densities were almost equal between the two land-use areas (Table 1). Basal area differences in the two areas were greater in magnitude in 2001 than 2015 and the differences in structure between the high-intensity and low-intensity land-use areas has diminished over time (Table 1). The entire LFDP-wide trend, in both land-use areas, is decreasing in basal area, with about a 50% reduction in host-tree basal area over the study period.
Liana population changes
Between 2001 and 2015, the number of liana stems increased in all 20 quadrats in both land-use areas in the LFDP (Figure 1). However, the increases were greater in the high-intensity land-use area. A repeated-measures analysis of variance (ANOVA) initially picked up statistical differences in liana abundance between the a priori land-use areas over time (F = 7.44, dfn = 1, dfd = 19, P = 0.0134). The glmm confirmed these differences (model fit (ω2) = 0.958, model deviance = 1941, df = 554, P << 0.001). Time was significant as a fixed effect in the model, and there was a significant interaction between land-use intensity and time, but land-use intensity alone was not significant. Including two random effects, one for species and one for quadrat improved model fit, with the effect of species having a slighter bigger impact on the model fit (model 3 in Table 2).
Table 2. Model selection for the effect of past land-use intensity, year, and quadrat (model 1), past land-use intensity, year and species (model 2), and past land-use history, year, quadrat and species on liana abundances in the LFDP, fit to 554 observations (i.e. total degrees of freedom). The final fitted was model 3 (ω2 = 0.958), with linear equation: log (liana abundace) = 0.13 (low − intensity land use) − 1.62 (year2001) + 0.75 (low − intensity land use*year2001) − 0.34.

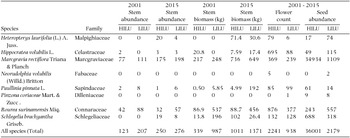
Liana biomass also increased over time, again with the greater increase in the high-intensity land-use area (total biomass for eight liana species are shown in Table 3). In 2001, liana biomass in the high-intensity land-use area of the LFDP was 1.22 Mg ha−1; by 2015 this rose to 3.39 Mg ha−1, an increase of 2.17 Mg ha−1 (177%). In the low-intensity land-use area in 2001 liana biomass was 3.14 Mg ha−1; by 2015 it was 4.17 Mg ha−1, an increase of 1.03 Mg ha−1 (33%).
Table 3. Liana abundances (≥1 cm liana stem at 1.3 m height) for the two census of the liana community (2001 and 2015) and total flower presence-absence basket counts and seed reproduction counts from 120 phenology baskets in the LFDP for the period (2001–2015) by past land-use intensity in the Luquillo Forest Dynamics Plot, Puerto Rico. Marcgravia rectiflora is the most abundant liana in the plot and exemplifies the described dynamic, where abundance and biomass rapidly, and disproportionately increased over time in the high-intensity land-use area and seed production was far greater in the high-intensity land-use area (HILU) than low-intensity land-use area (LILU).
In quadrats in the low-intensity land-use area, the sampled liana community occupied on average a greater proportion of individuals for each host species and a larger proportion of the total trees in the community (Figure 2). We examined trends in liana occupancy on trees in the assessed quadrats for the 12 dominant tree species in the host-tree community. In 2001, 25% of trees (183 tree individuals) were occupied by lianas while in 2015 this had increased to 44% of trees (405 individuals had one or more lianas present on the tree stem at 1.3 m height). In the community of the 12 dominant host-tree species within the study area, liana occupancy increased by roughly 50% over the 14-year study period (Figure 2).
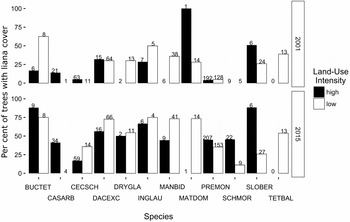
Figure 2. Per cent of trees hosting lianas, by species for the two censuses (2001 and 2015) in the Luquillo Forest Dynamics Plot, Puerto Rico. Numbers above bars correspond to the number of individuals in the tree community censused for that species, as the tree community changed over the 14 y between censuses. The high-intensity land-use area is shown in black, and the low-intensity land-use area is shown in white. Species codes correspond to the combined first three letters of tree genus and species: BUCTET – Buchenavia tetraphylla, CASARB – Caseria arborea, CECSCH – Cecropia schreberiana, DACEXC – Dacryodes excelsa, DRYGLA – Drypetes glauca, INGLAU – Inga laurina, MANBID – Manilkara bidentada, MATDOM – Matayba domingensis, PREMON – Prestoea acuminata var. montana, SCHMOR – Schefflera morototoni, SLOBER – Sloanea berteroana, TETBAL – Tetragastris balsamifera. Figure amended from Rice et al. (2004).
Despite increases in liana stem numbers, biomass and the number of trees occupied by lianas between 2001 and 2015, the canopy coverage of lianas did not appear to increase. In the 2001 census, the percentage of trees with 1–25% liana coverage was 13% (98 individuals), 26–50% was 3% (22 individuals), 51–75% was <1% (2 individuals) and 76–100% was <1% (2 individuals). By 2015, the percentage of trees in the same liana coverage categories was 14% (138 individuals), 6% (56 individuals), 3% (32 individuals) and 0% (0 individuals), correspondingly. These results indicate that there was little change in the qualitatively assessed canopy coverage of lianas in both the high-intensity and low-intensity land-use areas.
Liana flower and seed production
Liana flower and seed production counted in the phenology baskets between 2001–2015 paralleled changes in liana stem numbers. Flower and seed production was consistently greater in the high-intensity land-use area of the LFDP over this period (Table 1). Chi-square contingency tests revealed significant differences for liana reproductive trends, in both flowering and fruiting (liana fruits: χ2 = 280, df = 7, P << 0.01; liana seeds: χ2 = 10097, df = 7, P << 0.01). In these analyses, the abundance of Marcgravia rectiflora made the principal difference, weighting heavily in the statistical analyses, in that it was 10 times more abundant than the next most common species. Over the whole period of collections form the phenology baskets from 2001 to 2015, 34,934 seeds of M. rectiflora were collected in the high-intensity land-use portion of the LFDP, while only 1109 were collected in the low-intensity land-use area.
DISCUSSION
The hurricanes in 1989 (Hugo) and 1998 (Georges) reduced adult tree stem density (>10 cm dbh) between 2001 and 2015 in the 20 quadrats used for this liana study. After both hurricanes there was an increase in tree stem density of stems ≥1, <10 cm dbh in response to the open forest canopy. The number of tree stems ≥1 <10 cm dbh stems gradually decreased as the canopy closed and understorey stems died or grew larger to be >10 cm dbh and with crowns reaching into the canopy. After our study began, in 2001 (following Hurricane Georges in 1998), liana density, biomass, occupancy rates of trees, and flower and fruit production increased in all study quadrats, and to a greater extent in forest quadrats with a higher-intensity of past land use.
The ANOVA and the glmm both showed statistical differences between the two areas of differing land-use intensity over time. This suggests a direct response by lianas (as with trees) to hurricane effects: with initially a reduction of density and biomass of lianas as a result of damage to the forest canopy, when many trees lost branches and lianas fell to the ground or died after leaf loss, followed by increases in density, biomass and reproduction due to liana regrowth, assisted by the increase in density of small trees as support for lianas to climb. As forest structure matured, the larger trees with regrown crowns hosted larger lianas. In addition, the past land also influenced liana populations as more trees were damaged in the high-intensity land-use areas of the plot because of the greater susceptibility to damage of the tree species that grew and the greater openness of the damaged canopy in that part of the LFDP (Canham et al. Reference CANHAM, THOMPSON, ZIMMERMAN and URIARTE2010, Uriarte et al. Reference URIARTE, CANHAM, THOMPSON, ZIMMERMAN, MURPHY, SABAT, FETCHER and HAINES2009).
Due to the confounding effects of hurricane and historical land-use in the El Yunque forest at our site, although we see an increase in liana numbers and biomass such as reported for other Neotropical sites (Phillips et al. Reference PHILLIPS, MARTÍNEZ, ARROYO, BAKER, KILLEEN, LEWIS, MALHI, MENDOZA, NEILL and VARGAS2002), we cannot attribute the increase of lianas in our study to the same causes as in other forests (i.e. shifting forest demographics due to carbon dioxide fertilization and climate change). However, our study does show that in the younger forest area with a higher intensity of past land-use, increases in liana density and biomass accompanied a dynamic tree community, paralleling the increases in lianas and accelerating tree dynamics in other Neotropical forests (Phillips Reference PHILLIPS1996, Wright Reference WRIGHT2005). Additionally, stable dynamics in liana abundances have also been more recently documented over a similar time period in the well-conserved Yasuní Forest Dynamics Plot (Smith et al. Reference SMITH, QUEENBOROUGH, ALVIA, ROMERO‐SALTOS and VALENCIA2016), suggesting that increases in liana abundance stabilize with forest age, as we found in the area of low-intensity land-use in this study. The distribution of percentages of lianas in the tree crowns (i.e. liana canopy cover) was relatively stable from 2001 to 2015, most likely due to the stability of incoming solar radiation (i.e. energy) and the tendency for lianas to fill canopy space, regardless of liana density and biomass, with their sprawling growth habit once they establish in the canopy.
Lianas likely increased over the whole plot following the hurricanes, depending upon the location of tree and liana damage, followed by recovery due to increased light to the forest understorey. As the tree community and forest structure recovered, our results revealed a faster increase in lianas and higher flower and seed production in the high-intensity land-use area, underlying the overall effects of the hurricanes. This probably occurred for two reasons. First, the higher-intensity land-use area was still recovering from land use before 1934, and lianas were probably already increasing here as a result of this human disturbance, since increases in lianas abundances have been well-documented to persist for up to 50 y post-disturbance (Chazdon Reference CHAZDON and Chazdon2014, DeWalt et al. Reference DEWALT, SCHNITZER and DENSLOW2000, Letcher & Chazdon Reference LETCHER and CHAZDON2009, Tymen et al. Reference TYMEN, RÉJOU‐MÉCHAIN, DALLING, FAUSET, FELDPAUSCH, NORDEN, PHILLIPS, TURNER, VIERS and CHAVE2016). Second, the second hurricane (Hurricane Georges in 1998) caused more damage in the high-intensity land-use area (Canham et al. Reference CANHAM, THOMPSON, ZIMMERMAN and URIARTE2010, Hogan et al. Reference HOGAN, ZIMMERMAN, THOMPSON, NYTCH and URIARTE2016, Ogle et al. Reference OGLE, URIARTE, THOMPSON, JOHNSTONE, JONES, LIN, MCINTIRE, ZIMMERMAN, Clark and Gelfand2006, Uriarte et al. Reference URIARTE, CANHAM, THOMPSON, ZIMMERMAN, MURPHY, SABAT, FETCHER and HAINES2009), initially causing more tree branch and canopy liana loss in this area.
This was followed by more seedlings and faster growth of lianas and trees as the forest recovered from the hurricane, and was greater than in the low-intensity land-use area where the initial hurricane damage was less severe. The explanation is, that due to past land-use effects (i.e. clearing of the forest) the tree community in the high-intensity land-use area is still dominated by more secondary-forest species, with smaller stature and lower wood densities, which are more damaged by hurricanes than the native tree community, which is highly adapted to resist hurricane damage (Basnet et al. Reference BASNET, SCATENA, LIKENS and LUGO1993, Zimmerman et al. Reference ZIMMERMAN, EVERHAM, WAIDE, LODGE, TAYLOR and BROKAW1994). The lianas in the high-intensity land-use area would then exhibit in the study period a two-fold response, to the high-intensity land-use and the consequential effect on forest tree distributions to the combined effect of both hurricanes (Hogan et al. Reference HOGAN, ZIMMERMAN, THOMPSON, NYTCH and URIARTE2016). As the low-intensity land-use area suffered less damage from both Hugo and Georges, there was less light in the understorey and, therefore, a slower and more limited response from the lianas.
The liana community recovered quickly in the high-intensity land-use area. In only 14 y, liana abundance had rebounded to levels previously found in the low-intensity land-use area of the LFDP. However, liana biomass remained greater in the low-intensity land-use area. It was probably greater in the low-intensity land-use area before the hurricanes and was less affected by the second hurricane than in the high-intensity land-use area. Interestingly, despite presumed less disturbance by the second hurricane and greater biomass in the low-intensity land-use area, flower and seed production were greater in the high-intensity land-use area, where, evidently, conditions favour both liana growth and fecundity (mainly for M. rectiflora, however).
In a similar study comparing changes in liana abundances across a gradient of logging disturbance in Costa Rican wet forest, Yorke et al. (Reference YORKE, SCHNITZER, MASCARO, LETCHER and CARSON2013) described a 15% increase in liana abundance and a 20% increasing liana basal area in an old-growth area, and declining liana abundances in all selectively logged areas, over an 8-year interval. This contrasts from our findings, in that we report increases in basal area in the old-growth area of the Puerto Rican forest, with no change in liana abundance. Additionally, there seem to be inherent differences in the short-term response of lianas to direct human disturbance (i.e. logging) and natural disturbance (i.e. hurricanes), even though in our study hurricane disturbance effects are confounded by the lasting effects of past land use.
In conclusion, this study illustrates how lianas respond to disturbance in a tropical forest and how human and natural disturbances interact. In our forest, lianas responded to multiple hurricane disturbances with increased abundance, growth, tree occupancy and reproductive effort. With forest regrowth, these responses by lianas led to a greater increase in tree occupancy biomass of lianas in the high-intensity land-use area of younger forest, when compared with the low-intensity land-use area of older forest. Finally, the study illustrates a positive feedback between human and natural disturbance leading to altered successional trajectories over the long term and more variable short-term forest dynamics (Chazdon Reference CHAZDON and Chazdon2014, Scatena et al. Reference SCATENA, BLANCO, BEARD, WAIDE, LUGO, BROKAW, SILVER, HAINES, ZIMMERMAN, Brokaw, Crowl, Lugo, McDowell, Scatena, Waide and Willig2012). The area that was more disturbed by humans was more susceptible to hurricane disturbance, potentially perpetuating faster dynamics of both trees and lianas, a state manifested elsewhere in the Neotropics where forest dynamics are faster and lianas more abundant. These results have significant implications for the likelihood of successful recovery of trees in liana-infested secondary forests in the tropics.
ACKNOWLEDGEMENTS
We kindly acknowledge Stefan Schnitzer, the anonymous reviewers, and the editor for comments which greatly improved the manuscript. This research was supported by grants DEB 0080538 and DEB 1239764 from NSF to the University of Puerto Rico-Río Piedras for the Luquillo Long-Term Ecological Research Program. The U.S. Forest Service (Dept. of Agriculture) and the University of Puerto Rico gave additional support. We additionally acknowledge NSF Research Experience for Undergraduate (REU) program (NSF 552567 and 602642), which provided logistical support to SM. Funding was provided by the NSF ‘Bridge to the Doctorate Program’ to JAH during the 2015–2016 academic year, while he was at the University of Puerto Rico-Río Piedras (HRD-1139888). Lastly, we thank the support staff of the Luquillo LTER program office (UPR-RP) and El Verde Research Station.






