I. INTRODUCTION
Thermoelectric materials have been used for power generation using heat sources ranging from thermal radiation from Po-238 nuclear fuel in the NASA RTG program (Scock, Reference Scock1993; Bennett, Reference Bennett and Rowe1995) to energy harvesting from a small temperature gradient of a few degrees (Bierschenk, Reference Bierschenk, Priya and Inman2009; Snyder, Reference Snyder, Priya and Inman2009). Automotive exhaust gas from spark ignition engines has an average temperature around 500 °C, and the coolant temperature during operation is around 100 °C. Currently, no thermoelectric-based waste heat recovery system is employed in production vehicles. To generate maximum power in the 100–500 °C range, several thermoelectric materials were considered good candidates and include: PbTe (LaLonde et al., Reference LaLonde, Pei, Wang and Snyder2011; Zhang et al., Reference Zhang, Wang, Zhang, Liu, Yu, Wang, Wang, Ni, Chen and Ren2012), clathrates (Kuznetsov et al., Reference Kuznetsov, Kuznetsova, Kaliazin and Rowe2000; Poon, Reference Poon, Tritt, Willardson and Weber2001; Nolas, Reference Nolas and Rowe2005), half-Heusler (Bhattacharya et al., Reference Bhattacharya, Pope, Littleton, Tritt, Ponnambalam, Xia and Poon2000; Joshi et al., Reference Joshi, Yan, Wang, Liu, Chen and Ren2011) and skutterudites (Sales et al., Reference Sales, Mandrus and Williams1996). These materials have similar figures of merit, zT (zT = s 2 T/ρk, where s is the Seebeck coefficient, ρ is the electrical resistivity, k is the thermal conductivity and T is the temperature in Kelvin), with values reported to be 1.0–1.8 with ±20% uncertainty. PbTe and clathrate materials generally have power factors (s 2/ρ) that are about half that of optimally doped skutterudites and half-Heuslers, but PbTe and clathrates have much lower total thermal conductivity as compared with skutterudites and half-Heuslers.
Selection of a material for prototype thermoelectric generators from the literature, which were solely from the laboratory-based research, required considerations of transport properties data reliability, reproducibility, and other practical criteria, including fracture strength and constituent element abundance. The specific vehicle operating conditions and cost restrictions for high-volume automobile production were used to further narrow down candidate materials in a down-select process. For example, PbTe was a good material under constant temperature gradient in the NASA RTG application. However, its brittle nature and poor thermal shock resistance made it unsuitable for automotive applications where repeated thermal cycling and harsh vibrational loading are encountered. Other materials such as half-Heusler and clathrates contain large quantities of expensive elements such as Hf and Ge, respectively. Both are good thermoelectrics in the automotive exhaust application temperature range, although they are cost-prohibited for the intended applications. Skutterudites have been selected as the candidate materials for automotive waste heat recovery because of their high zT, good mechanical properties, and high thermal shock resistance in the waste heat exhaust temperature range (Salvador et al., Reference Salvador, Yang, Shi, Wang, Wereszczak, Kong and Uher2009). Cost–benefit analysis also showed skutterudites could meet the cost targets (dollar per watt) for large-scale automotive applications.
Skutterudite (CoSb3), in its pure form, has very good electronic transport properties and relatively high thermal conductivity. The skutterudite crystal structure has an interstitial void (or “cage”) that can be filled with impurity atoms, as seen in Figure 1. Filled skutterudites are good examples of the so-called phonon-glass-electron-crystal (PGEC) (Slack and Tsoukala, Reference Slack and Tsoukala1994; Nolas et al., Reference Nolas, Morelli and Tritt1999) materials. The main concept of PGEC is to have thermoelectrics that can maintain good electrical performance (high-power factor PF = s 2/ρ) , and lower thermal conductivity (k) by enhancing phonon scattering with the filler atoms. Single and multiple filler atoms selected from La, Ce, Yb, Ba, Ca, Sr, Ca, In, Tl, Na, etc. have been used to form partially filled and fully filled skutterudites.

Figure 1. (Color online) Crystal structure of skutterudite, with the filler atoms in cyan, the Sb atoms in tan, and the Co atoms in the dark blue octahedra. The large cages are centered on the corners and in the center of the unit cell.
In the past 20 years, incremental improvements in zT have been made in both n- and p-type skutterudites. However, some filler atoms do enhance performance and some that have good initial performance are not stable with thermal cycling (Li et al., Reference Li, Tang, Zhang and Uher2009). While many types of filler seem to improve zT compared with CoSb3, a few filler atoms such as Ce, Yb, and Ba seem to show both expected enhancement in zT and long-term stability. The n-type material filled with Yb (Nolas et al., Reference Nolas, Kaeser, Littleton and Tritt2000) has been demonstrated to have zT above 1.2 at 500 °C. Recently, double- and triple-filling of n-type materials (Shi et al., Reference Shi, Cho, Bai, Yang, Wang, Chi, Salvador, Zhang, Chen and Wong-Ng2010; Salvador et al., Reference Salvador, Cho, Ye, Moczygemba, Thompson, Sharp, König, Maloney, Thompson, Sakamoto, Wang, Wereszczak and Meisner2013) have been reported to yield zT of 1.1–1.4 and higher. Combination of filler atoms with different oscillation frequencies (e.g., Ba and Yb) have been shown to be more effective than atoms with similar oscillation frequencies (e.g., La and Ce). In the p-type materials, most reports showed maximum zT between 0.8 and 1.0 (Morelli et al., Reference Morelli, Caillat, Fleurial, Borshchevsky, Vandersande, Chen and Uher1995; Tang et al., Reference Tang, Zhang, Chen, Goto and Hirai2005). To match the performance of n-type materials, significant research efforts have been put into developing a better p-type material (Tang et al., Reference Tang, Hanus, Chen and Snyder2015). The replacement of Co with Fe/Ni was suggested by Singh and Du (Reference Singh and Du2010) and p-type materials using this concept showed good properties and zT (Tan et al., Reference Tan, Wang, Yan, Li and Tang2012). Another alternative p-type composition uses didymium (Di), a natural mixture of Pr and Nd (Rogl et al., Reference Rogl, Grytsiva, Bauerb, Rogla and Zehetbauerc2010), to replace Ce and La in the Fe/CoSb3 system.
In this study, the selected n-type material was Ba–Yb double-filled CoSb3. The p-type material had both Di filling and Fe/Ni substitution for Co. Both materials were candidate compositions for the vehicle waste heat recovery applications. To produce reliable skutterudite modules for the thermoelectric generator, a large quantity of materials needs to be processed with small variations in compositions, properties and performance. The main challenge is to scale up the material production from laboratory scale (5–10 g) to prototype scale (>100 g) and maintain the consistency and quality in transport properties. The skutterudite materials were prepared using an industrial scale melt-spin process. Powders were heat treated and prepared (milled and sieved) at Marlow Industries and General Motors prior to the consolidation. This study is focusing on the X-ray diffraction (XRD) and neutron diffraction of the skutterudite powders used to densify materials for thermoelectric modules. Although the crystal structure of skutterudite is known, XRD and neutron diffraction data on production-ready materials are not available. It is important to provide a comprehensive data and analyses using both XRD and neutron diffraction methods. Detailed analyses of the crystal structures of the n- and p-type skutterudites are presented.
II. EXPERIMENTAL
A. Synthesis of skutterudite materials
The materials were processed at Molycorp in the following manner. For the n-type skutterudite, 100 g charges with nominal compositions Yb0.08Ba0.07Co4Sb12.12 and Yb0.13Ba0.10Co4Sb12 were synthesized by combining the elements in their desired stoichiometric ratios in a carbon-coated fused silica tube. The charged tube was then sealed under a reduced atmosphere of 50 × 10−2 Torr, followed by induction melting up to 1350 °C for a 3-min period, under an 800 Torr Argon back pressure. The resulting ingots were then melt-spun under Ar by heating them to 1350 °C and then ejecting them (with a 250 Torr pressure differential) onto a rotating quench wheel.
For the composition Yb0.08Ba0.07Co4Sb12.12, a wheel speed of 5 m s−1 was used for a slow quench and a second wheel speed of 22 m s−1 was used for a fast quench. The powders were subsequently annealed at 700 °C for 5 days.
The p-type samples had two compositions, Nd0.1Pr0.3Fe4−x Co x Sb12 (x = 0.5) and Nd0.18Pr0.5Fe3.4Ni0.6Sb12. They were synthesized similarly to the n-type material. The ribbons were then annealed at 560 °C for 5 days. At Marlow Industries, synthesis was completed by milling and hot-pressing melt-spin powders from Molycorp. Melt-spun ribbons are shown in Figures 2(a) and 2(b).

Figure 2. (Color online) (a) Picture of the melt-spun ribbons and (b) scanning electron microscopy image of the cross-section of melt-spun ribbon.
B. XRD measurements and analysis methods
Continuous Θ-2Θ scans were performed on a 4-axis diffractometer from nominally 10–120°2θ in under 0.5°2θ min−1. Using CoKα 1 radiation (λ = 1.788 965 Å) and an energy-dispersive point detector. The powders were oscillated ±2 mm. A search–match was conducted for each data set using the Jade 9.4.5 software and the ICDD PDF 2011 database.
C. Neutron diffraction measurements and analysis methods
Time-of-flight (TOF) neutron diffraction data were collected at the Spallation Neutron Source (SNS) on the POWGEN powder diffractometer (beamline 11a) (Huq et al., Reference Huq, Hodges, Gourdon and Heroux2011). For each composition, approximately 4–8 g powder were loaded into a vanadium sample can, and data were collected at 23 °C, with a d-spacing range of 0.4–3.6 Å.
X-ray and neutron data were analyzed together in joint refinements in order to take advantage of the different atomic contrasts available with the two different probes. Data were refined with GSAS (Larson and Von Dreele, Reference Larson and Von Dreele2000) and EXPGUI (Toby, Reference Toby2001) using the TOF (Von Dreele et al., Reference Von Dreele, Jorgensen and Windsor1982) and pseudo-Voigt profile functions for neutron and X-ray data, respectively, and fitting the background with a reciprocal interpolation function.
III. RESULTS AND DISCUSSION
A. Phase analysis
Figures 3 and 4 show the graphic results of the joint Rietveld refinements for the neutron and XRD data, respectively. For all four compositions, the diffraction patterns indicate that the major phase has the skutterudite structure. Skutterudite has body-centered cubic symmetry, crystallizes in the space group Im
![]() $\bar 3$
, and consists of a framework of corner-sharing (Fe, Co, Ni)Sb8 octahedra. Within this framework are large icosahedral “cages”, of the order of 100 Å3 in volume, which contain the filler atoms, which donate charge and lower the lattice thermal conductivity.
$\bar 3$
, and consists of a framework of corner-sharing (Fe, Co, Ni)Sb8 octahedra. Within this framework are large icosahedral “cages”, of the order of 100 Å3 in volume, which contain the filler atoms, which donate charge and lower the lattice thermal conductivity.
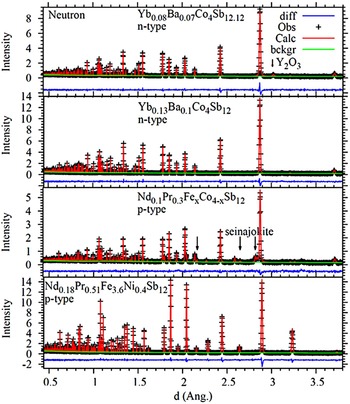
Figure 3. (Color online) Neutron diffraction patterns of two Yb–Ba filled n-type and two Nd–Pr filled p-type skutterudite materials, with the major Y2O3 and seinajokite (FeSb2) secondary phase peaks marked with arrows.

Figure 4. (Color online) XRD patterns of two Yb–Ba filled n-type and two Nd–Pr filled p-type skutterudite materials, with the major Yb2O3 and seinajokite (FeSb2) secondary phase peaks marked with arrows.
In addition to the primary skutterudite phase, secondary phases were identified in two of the compositions studied. For the n-type composition Yb0.08Ba0.07Co4Sb12.12, a trace of Yb2O3, approximately 1 wt%, was found. The materials processing and sintering were not conducted in high vacuum or inert gas with low oxygen partial pressure. It is common to find oxides in skutterudites. In some cases, oxide particles help to reduce thermal conductivity. For the p-type Fe–Co composition, a secondary phase of seinajokite (Powder Diffraction File PDF#04-003-2029), i.e. (Fe, Co)Sb2, was present at ~12 wt%, along with a trace of another phase, the peak positions of which are consistent with elemental antimony (PDF#04-010-9926). The partial formation of Fe-rich seinajokite and elemental antimony in Fe-rich skutterudite has been previously reported for Co-based skutterudites (Peng et al., Reference Peng, Yang, Zhang, Song and Chen2004).
These secondary phases were not observed in the Fe–Ni skutterudite with composition (Nd0.18Pr0.51)(Fe3.6Ni0.4)Sb12, despite the high Fe-content. Previous research by several of the authors of this work on Ni-based skutterudites has suggested that the addition of filler atoms can stabilize the skutterudite structure and prevent the formation of seinajokite and elemental antimony.
B. Skutterudite lattice
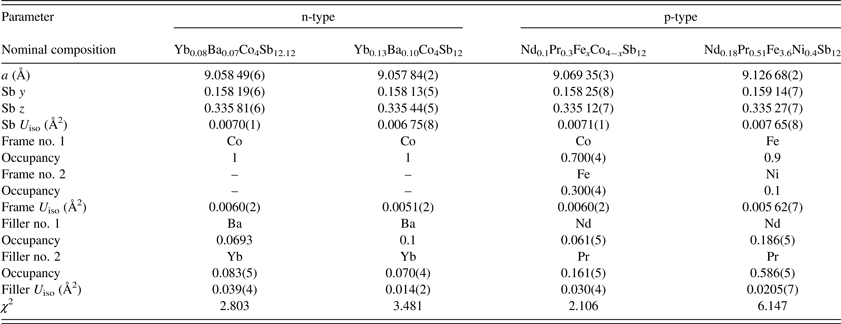
For the compositions studied, the lattice parameter of the skutterudite phase was found to be dependent on the framework atoms, as seen in Table I. For the two n-type compositions, which both have frameworks composed of only Co and Sb, the lattice parameters are effectively identical, with the difference being within estimated error levels (Toby, Reference Toby2006). For the p-type material with a minor fraction (roughly 30%) of the Co replaced with Fe, the lattice parameter is slightly larger, approximately 0.01 Å or 0.12%. For the p-type material with all of the Co replaced with a mixture of 90% Fe and 10% Ni, the lattice parameter is significantly larger, approximately 0.07 Å or 0.76%, as compared with the n-type materials. The finding that compositions with greater Fe content have larger lattice parameters is consistent with the larger atomic size of Fe (1.32 Å) relative to Co (1.26 Å) (Cordero et al., Reference Cordero, Gómez, Platero-Prats, Revés, Echeverría, Cremades, Barragán and Alvarez2008).
Table I. Refined skutterudite structural parameters. Numbers in parentheses are estimated standard deviations based on the fit, which are known to be underestimates of the true error.
C. Framework atoms
The skutterudite structure has high-symmetry, which helps to limit the number of atomic parameters to be refined. The Fe/Co/Ni and filler atoms sit on fixed special positions (8c and 2a Wyckoff sites, respectively); Sb sits on a 24 g site (0, y, z), with the y and z parameters variable. Owing to the high potential for correlation among atomic parameters, however, some constraints were necessary in the refinements to avoid instabilities. Atoms occupying the same position were constrained to have equal displacement parameters. Framework atom positions, i.e. Sb and Fe/Ni/Co, were assumed to be fully occupied. Some additional constraints were necessary on the filler atom occupancies, as will be discussed below.
Neutron diffraction allowed refinement of the Fe:Co ratio for the p-type composition. Fe and Co have quite poor diffraction contrast for X-rays (atomic numbers, Z, of 26 and 27, respectively), and generally cannot be distinguished with X-rays alone. However, Fe and Co have much better contrast for neutrons (bound coherent scattering lengths, b c, of 9.45 and 2.49 fm, respectively (Dianoux and Lander, Reference Dianoux and Lander2003). From the joint X-ray/neutron refinements, the skutterudite phase was found to be Co-rich, with an Fe:Co ratio of 0.3:0.7. This composition also had a secondary phase of seinajokite, (Co,Fe)Sb2, which was found to be Fe-rich, with an Fe:Co ratio of 0.8:0.2. Considering the fractions of the two phases, the overall Fe:Co ratio was found to be approximately 0.4:0.6. On the other hand, Fe and Ni have quite poor diffraction contrast with both X-rays (Z = 26 and 28, respectively) and neutrons (b c = 9.45 and 10.3 fm, respectively). Therefore, for the nominal composition (Nd0.18Pr0.51)(Fe3.6Ni0.4)Sb12, the Fe and Ni occupancies could not be independently refined and were fixed at the nominal values.
For all compositions, the displacement parameters of the framework atoms were similar. The average isotropic displacement parameters, U iso, are 0.0057(4) Å2 for the Fe/Co/Ni atoms, and 0.0071(3) Å2 for the Sb atom, consistent with previous reports (e.g., Chakoumakos et al., Reference Chakoumakos, Sales, Mandrus and Keppens1999). In the same way, the positional parameters of the Sb atom were similar for all compositions. The average refined Sb y and z values are 0.1584(4) and 0.3354(3), respectively.
D. Filler atoms
Compared with the framework atoms, the atomic parameters of the filler atoms were much more sensitive and susceptible to correlation. However, general trends in these parameters provide useful information. The filler atoms sit on a special position [Wyckoff position 2a (0,0,0)] with no free atomic coordinates to refine. The isotropic displacement parameters of the two filler atoms were fixed to be equal. The refined U iso values for the filler atoms are almost an order of magnitude larger than the displacement parameters for the framework atoms, with an average across all four samples of 0.026(9) Å2, similar to previous reports (Chakoumakos et al., Reference Chakoumakos, Sales, Mandrus and Keppens1999). The larger displacement parameters are consistent with a greater degree of positional disorder, or rattling, of the filler atoms. The size of the cage (with a volume of 94–97 Å3, depending on the lattice parameter) is roughly an order of magnitude larger than the size of the filler atom, leaving room for motion of the filler atom inside.
Distinguishing the filler atoms to refine their occupancies poses a special challenge. For the p-type samples, praseodymium and neodymium have very poor diffraction contrast with X-rays (Z = 59 and 60, respectively). Although the diffraction contrast with neutrons [b c = 4.58 and 7.69 fm, respectively (Dianoux and Lander, Reference Dianoux and Lander2003)] is somewhat better, the small overall degree of filling reduces the signal sufficiently that the Nd and Pr site occupancies could not be independently refined with any success. Therefore, it was assumed that the Nd/Pr ratios would not stray far from the nominal values, and the occupancies were restrained accordingly. This assumption is reasonable, as Nd and Pr have similar chemical behavior and are not easily separated, to the extent that naturally occurring mixtures of Nd and Pr were originally thought to be a single element, Di, in the 19th century and were indicated as such in Mendeleev's periodic table (Mendelejeff, Reference Mendelejeff1869).
Despite these restrictions, useful information may be drawn from the praseodymium and neodymium filler atom occupancies. For the p-type material with Fe and Co framework atoms, the filler atom occupancies refined to approximately half of the nominal values. In contrast, for the p-type material with Fe and Ni framework atoms, the filler atom occupancies refined to values close to the nominal value, indicating that all of the filler atoms are going into the skutterudite structure. This held true even when disturbing the starting values far from the nominal values. The difference in behavior of the two p-type compositions likely relates to the larger lattice parameter of the Fe/Ni skutterudite [9.126 68(4) vs. 9.06935(3) Å for the Fe/Co p-type skutterudite].
The Ba and Yb filler atoms for the n-type materials exhibit better contrast with both X-rays (Z = 56 and 70, respectively) and neutrons [b c = 5.07 and 12.41 fm, respectively (Dianoux and Lander, Reference Dianoux and Lander2003)]. However, the Ba and Yb occupancies are still too highly correlated to be refined independently. Attempts to do so resulted in a non-physical negative occupancy for Yb. Owing to this observation, along with the presence of an Yb2O3 secondary phase in one of the samples, the filler atom occupancies were restrained by fixing the Ba occupancy at the nominal value and refining the Yb occupancy. For the composition Yb0.08Ba0.07Co4Sb12.12, the Yb occupancy [0.083(5)] refined to the nominal value. For the composition Yb0.13Ba0.1Co4Sb12, the Yb occupancy refined to approximately half [0.070(4)] of nominal. For both compositions, the total filling fractions are similar (0.15 and 0.17), despite nominally having different levels of additions (0.15 and 0.23). Combined with the existence of the Yb2O3 secondary phase, this suggests that there is a maximum on the amount of filler atoms that can be put in the skutterudite cages of CoSb3.
IV. CONCLUSIONS
From the diffraction data, several potentially useful points may be observed. First, there is a limit to the amount of Fe that can be incorporated into the skutterudite structure with these fillers. Beyond that level, the excess Fe enters a seinajokite secondary phase. The amount of fillers present may also have an effect on the amount of Fe that can be incorporated. There appears to be an upper limit on the degree to which the cages may contain filler atoms, with the excess filler atoms forming secondary phases.
Considering the refined filler atom occupancies for all compositions, a consistent relationship appears between the lattice parameter and the total filler occupancy, despite differences in the types of framework and filler atoms, as may be seen in Figure 5. The total fraction of cage filling depends linearly on the lattice parameter. This suggests that a key determining factor for the degree of filling is a steric effect. A larger lattice can better accommodate the filler atoms.
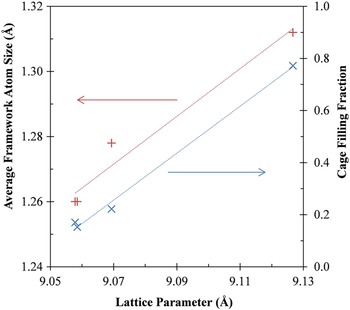
Figure 5. (Color online) Average framework atom size (red +) and total cage filling fraction (blue ×) as a function of lattice parameter.
Similarly, the lattice parameter is approximately linearly related to the weighted average of the framework atom sizes, as seen in Figure 5. Combining these points suggests a method of optimizing the cage filling fraction by adjusting the framework atom composition, which may ultimately allow the properties of these skutterudite thermoelectric materials to be better tuned for automotive waste heat recovery applications.
ACKNOWLEDGEMENTS
The authors acknowledge the support of the Assistant Secretary of Energy Efficiency and Renewable Energy of the Department of Energy (DOE) and the Vehicle Technology Program. This work was supported by General Motors, Marlow Industries and by the Department of Energy under Award No. DE-FC26-04NT42278 and DE-EE0005432. Part of the none-user program work performed at ORNL was under a Work for Other (WFO) contract with General Motors. The User Program research conducted at ORNL's Spallation Neutron Source was sponsored by the Scientific User Facilities Division, Office of Basic Energy Sciences, US Department of Energy. Oak Ridge National Laboratory is managed by the UT Battelle LLC, for the Department of Energy under Contract No. DE-AC05000OR22725.








