I. INTRODUCTION
Over the past few decades, the application of various semiconductor photocatalysts in solar energy conversion and environmental purification has been investigated extensively (Hoffmann et al., Reference Hoffmann, Martin, Choi and Bahnemann1995; Han et al., Reference Han, Kambala, Srinivasan, Rajarathnam and Naidu2009; Ferrari-Lima et al., Reference Ferrari-Lima, de Souza, Mendes, Marques and Gimenes2015). Many well-known semiconductors including TiO2 (Yang et al., Reference Yang, Liu, Zheng, Yuan, Zhao, Waclawik, Ke and Zhu2009; Wu et al., Reference Wu, Fan, Liu and Hu2015) and ZnO (McLaren et al., Reference McLaren, Valdes-Solis, Li and Tsang2009; Shi et al., Reference Shi, Liang, Ma, Meng and Zhong2014) have been accepted as the most efficient photocatalysts. However, due to their large band gap, these photocatalysts are only active in the ultraviolet (UV) region, which is the obstacle for their practical applications. Therefore, on the one hand, much work has focused on the modification of these traditional photocatalysts; on the other hand, much effort has been made to develop other novel efficient photocatalysts. Recently, bismuth oxychlorides [BiOX(X = Cl, Br, I)] have been the subject of novel photocatalyst series because of their advanced photocatalytic activity, which can be attributed to their unique layered structures and high chemical stabilities (Zhang et al., Reference Zhang, Ai, Jia and Zhang2008; Li et al., Reference Li, Chen, Zhou and Shen2011a, Reference Li, Wang, Yao, Dang and Li2011b, Reference Li, Yu and Zhang2014, Reference Li, Zhang, Tang, Ying and Xu2015; Lin et al., Reference Lin, Huang, Long and Sun2014; Zhang et al., Reference Zhang, Wang, Sun, Jiang and Gao2015). The internal static electric field perpendicular to each layer in the structure can effectively separate electron–hole (e–h) pairs and the distance to surface for e–h moving is relatively minimized, which can induce a higher photocatalytic performance as result. As the representative photocatalyst of Bi-based oxychlorides, BiOCl reveals higher photocatalytic activity than TiO2 under UV irradiation (Chen et al., Reference Chen, Liu, Bagwasi and Shen2010; Zhang et al., Reference Zhang, Liu, Fan, Wang and Wang2013). However, similar to traditional semiconductor photocatalysts, BiOCl could not photodegrade organic dye under visible light because of wide band gap between 3.19 and 3.44 eV and some modification routes were applied for the purpose of extending light-responsive range into visible light (Li et al., Reference Li, Chen, Zhou and Shen2011a, Reference Li, Wang, Yao, Dang and Li2011b; Tan et al., Reference Tan, Zhu, Hojamberdiev, Xu and Liang2013; Tian et al., Reference Tian, Liu and Gong2013; Ao et al., Reference Ao, Tang, Wang and Wang2014; Xie et al., Reference Xie, Mao, Fan and Wang2014; Zhu et al., Reference Zhu, Hojamberdiev, Tan, Jin and Xu2014).
In recent years, it was realized that the series of bismuth oxychlorides with different stoichiometric ratio might be a novel candidate for effective visible-light-excited photocatalyst (Lin et al., Reference Lin, Huang, Huang, Wang and Shi2006; Gao et al., Reference Gao, Chakraborty, Yang and Lee2010; Xiao et al., Reference Xiao, Liu, Hu, Zuo, Nan, Li and Wang2012, Reference Xiao, Jiang and Zhang2013; Chen et al., Reference Chen, Fang and Tang2013). In 2006, Huang and co-workers reported the preparation of Bi3O4C1 with a band gap (E g = 2.79 eV) and demonstrated its visible-light-excited photocatalytic ability (Lin et al., Reference Lin, Huang, Huang, Wang and Shi2006). Their investigation suggests that different stoichiometric bismuth oxychlorides may improve optical properties and enhance photocatalytic performance under visible light. In 2012, Nan et al. prepared a series of bismuth oxychlorides with different stoichiometric ratio including Bi3O4C1, Bi12O17Cl2, Bi12O15Cl6, and Bi24O31Cl10 (Xiao et al., Reference Xiao, Liu, Hu, Zuo, Nan, Li and Wang2012). With the decrease of Cl percentage, light absorption was extended to visible-light region. In addition, Zhang et al. demonstrated that Bi12O17Cl2 nanobelts prepared by conventional hydrothermal method could efficiently oxidize benzyl alcohol (BA) into benzaldehyde under visible light (Xiao et al., Reference Xiao, Jiang and Zhang2013). The size of the nanostructures mentioned above mainly lies in several hundreds of nanometers and such target pollutant as bisphenol A, rhodamine B, pentachlorophenol, and BA were used. This series of photocatalysts have been seldom applied to degrade methyl orange (MO), the representative of azo dyes. Hence, there still remains a great challenge not only to design different stoichiometric bismuth oxychloride with well-defined nanostructure as photocatalyst, but also to investigate the correlation between nanostructure characteristics and their photocatalytic activity.
Herein, we confirm the feasibility to employ as-prepared Bi12O17Cl2 nanomaterial as photocatalyst to degrade MO under visible light. The MO photodegradation efficiency was achieved up to 96.9% in 90 min. In particular, the novel plate-stratiform nanostructure was firstly reported. For Bi12O17Cl2 photocatalyst, synthesis route and high MO degradation efficiency were reported for the first time. The dependency of superior visible-light-responsive photocatalytic activity on novel nanostructure was investigated.
II. EXPERIMENTAL
A. Synthesis
All chemicals (Sinopharm Chemical Reagent Co., Ltd.) were of analytical reagent grade and used as received without further purification. For a typical synthesis, Bi2O3 and Bi(NO3)3·5H2O were used as raw materials, and BiOCl appeared as an intermediate product. The synthesis of BiOCl was described as below. Firstly, 0.02 mole of Bi(NO3)3·5H2O was dissolved in 20 ml of glacial acetic acid (CH3COOH). After being magnetically stirred for 15 min, solution I was obtained. Secondly, NaCl (0.02 mole) and sodium acetate (CH3COONa, 0.03 mole) were put into deionized water (200 ml). The mixture solution was magnetically stirred for 40 min and solution II was obtained. Thirdly, solution I was added into solution II drop by drop and the mixture solution was magnetically stirred for 12 h, a white suspension was formed. Finally, the suspension was centrifugally separated and washed with alcohol and deionized water for three times, respectively. After being dried at 60 °C for 10 h, white BiOCl powder was collected. The Bi12O17Cl2 photocatalyst was prepared by a solid-state reaction as follows. As-prepared BiOCl powder (0.008 mole) and Bi2O3 powder (0.02 mole) were ground for 5 h and calcined at 600 °C in air for 12 h. The resulting yellow Bi12O17Cl2 product was collected and ground for 15 min before further characterization.
B. Characterization
Formation of Bi12O17Cl2 was confirmed by a Rigaku D/Max 2200 X-ray diffractometer (CuKα radiation λ = 1.5406 Å). Morphology of the Bi12O17Cl2 powder was observed by a field-emission scanning electron microscope (JSM-6700F). Microstructures of Bi12O17Cl2 sample were characterized by transmission electron microscopy (TEM, JEM-2100), high-resolution transmission electron microscope (HRTEM), and selected-area electron diffraction (SAED) with a 200 kV accelerating voltage. To confirm light absorption range of Bi12O17Cl2 sample, diffuse reflectance spectra (DRS) in wavelength of 300–800 nm was measured by UV–vis spectrophotometer (Lambda 35).
C. Photodegradation performance test
The photocatalytic activity of the obtained Bi12O17Cl2 sample was evaluated by degradation of MO under visible light. The photocatalyst-dye mixture solution was put in a quartz cell with a circulating water jack outside, which ensures the reaction proceeding at room temperature to prevent any thermal catalytic effect. The visible-light illumination was obtained by placing a 420 nm cut-off filter under a 300 W Xenon lamp. The concentration of initial MO solution is 10 mg l−1, and the photocatalyst concentration in the MO solution is 1 mg ml−1. Before irradiation, 50 ml suspension was ultra-sonicated for 10 min and magnetically stirred for 20 min, respectively, in dark for the achievement of adsorption–desorption equilibrium of MO on catalyst surfaces. During visible-light irradiation, about 5 ml suspension was taken from the reaction cell at given time intervals. All slurry samples containing catalyst were centrifuged for 5 min at the rate of 8000 rpm to remove the catalyst powder. MO concentration of the filtrate was determined using the UV–vis spectrometer, and the characteristic absorption of MO at 464 nm was used to monitor the photocatalytic degradation.
III. RESULTS AND DISCUSSION
A. Morphology and microstructure
The morphology and microstructure of the Bi12O17Cl2 sample was characterized by SEM and TEM. In Figure 1(a), it was observed that as-prepared Bi12O17Cl2 is of regular plate-stacking structure constructed of dozens of nanosheets, which are about 10–50 nm in thickness and 200–1500 nm in in-plane size. These nanosheets with smooth surface and edges are described as secondary nanosheets in this study. Furthermore, the microstructures of the well-defined primary nanosheets are observed by TEM at higher magnifications. As shown in Figure 1(b), the distinct contrast of light and dark at the edge of a typical secondary nanosheet reveals that the secondary nanosheet is actually consisted of a parallel array of ultrathin nanosheets, which are named as primary nanosheets. The thickness of the primary nanosheets was in several nanometers and their boundaries are marked as “A–A”, “B–B”, and “C–C” in the inset illustration [Figure 1(b)]. To sum up, the as-synthesized Bi12O17Cl2 nanomaterial was firstly observed as being composed of secondary nanosheets regularly arranged. And more significantly, the relatively thicker secondary nanosheets are plate-stacked of ultrathin primary nanosheets, which are parallelly aligned and closely superimposed. Herein, this distinct hierarchical Bi12O17Cl2 microstructure in our work is designated as a novel plate-stratiform nanostructure.

Figure 1. (colour online) (a) SEM and (b) TEM images of plate-stratiform nanostructured Bi12O17Cl2.
B. Crystal imaging and diffraction
The X-ray diffraction (XRD) pattern of as-prepared sample is shown in Figure 2, which can be indexed to tetragonal Bi12O17Cl2 with lattice parameters of a = 5.443 and c = 35.2 Å (JCPDS No. 37-0702). It can be seen that the sample is well crystallized. Meanwhile, few small peaks were unable to be indexed and denoted as impurity peaks. Further, the above-narrated plate-stratiform Bi12O17Cl2 nanostructure was studied by SAED patterns and HRTEM images. Figure 3(a) is the SAED pattern of the typical secondary nanosheet shown in Figure 2(b). It is indexed as three zone axes [011],
![]() $[0\bar 71]$
, and
$[0\bar 71]$
, and
![]() $[05\bar 3]$
of tetragonal Bi12O17Cl2 (JCPDS No. 37-0702). Three sets of diffraction spots are respectively allocated to three axes, which are identified by A, B, and C subscripts. In addition, arrays of other relatively weak diffraction spots could be explained by secondary diffraction principle, which is caused by the distinct plate-stratiform structure. The SAED pattern proves that the secondary nanosheet is stacked of several single-crystalline primary nanosheets and confirms the nature of layer-stacked structure. In the HRTEM image given in Figure 3(b), the interplanar distance of 0.272 nm is consistent with (200) planes, which are common to three sets of SAED diffraction spots marked in Figure 3(a). At the same time, a typical primary nanosheet is analyzed by HRTEM and SAED, as shown in Figure 4. Clear diffraction spots and lattice fringes reveal that the primary nanosheet is highly single-crystalline. Figure 4(a) is an ultrathin primary nanosheet located at the corner of a secondary nanosheet as pointed by the arrow. The corresponding SAED pattern [Figure 4(b)] is along [001], displaying (020), and (220) planes with an angle of 45°. In Figure 4(c), the lattice fringe spacing of 0.195 nm is consistent with (200) planes of tetragonal Bi12O17Cl2.
$[05\bar 3]$
of tetragonal Bi12O17Cl2 (JCPDS No. 37-0702). Three sets of diffraction spots are respectively allocated to three axes, which are identified by A, B, and C subscripts. In addition, arrays of other relatively weak diffraction spots could be explained by secondary diffraction principle, which is caused by the distinct plate-stratiform structure. The SAED pattern proves that the secondary nanosheet is stacked of several single-crystalline primary nanosheets and confirms the nature of layer-stacked structure. In the HRTEM image given in Figure 3(b), the interplanar distance of 0.272 nm is consistent with (200) planes, which are common to three sets of SAED diffraction spots marked in Figure 3(a). At the same time, a typical primary nanosheet is analyzed by HRTEM and SAED, as shown in Figure 4. Clear diffraction spots and lattice fringes reveal that the primary nanosheet is highly single-crystalline. Figure 4(a) is an ultrathin primary nanosheet located at the corner of a secondary nanosheet as pointed by the arrow. The corresponding SAED pattern [Figure 4(b)] is along [001], displaying (020), and (220) planes with an angle of 45°. In Figure 4(c), the lattice fringe spacing of 0.195 nm is consistent with (200) planes of tetragonal Bi12O17Cl2.

Figure 2. (colour online) XRD pattern of as-prepared Bi12O17Cl2 sample.

Figure 3. (colour online) SAED pattern (a) and HRTEM image (b) of a typical Bi12O17Cl2 secondary nanosheet.

Figure 4. (a) TEM image of a typical Bi12O17Cl2 primary nanosheet as pointed by the arrow; (b) the corresponding SAED pattern; and (c) HRTEM image.
C. Optical absorption
DRS is often used to characterize optical absorption property, which is considered as the criteria for photocatalytic activities of semiconductors. Figure 5 exhibits the UV–vis diffuse reflectance spectrum of as-prepared Bi12O17Cl2 nanomaterial, which indicates that light absorption originates from the UV region and extends up to visible-light region. The light absorption edge is up to 590 nm, consisting of yellow color of the Bi12O17Cl2 powder. The DRS measurement suggests that as-prepared Bi12O17Cl2 has the potential to be photoactive under visible-light irradiation. Meanwhile, the band gap energy of the Bi12O17Cl2 nanomaterial was calculated by the formula αhν = A(hν − E g) n/2, where α, ν, E g, and A are the absorption coefficient, light frequency, band gap energy, and a constant, respectively (Butler, Reference Butler1977). Besides, n is a constant that depends on the optical transition in a semiconductor. Optical transition of Bi12O17Cl2 is indirect and its n value is 4. From the plot of (αhν)1/2 vs. hν (inset of Figure 5), E g of Bi12O17Cl2 sample is estimated to be 2.1 eV by the intercept of the tangent to the plot.

Figure 5. (colour online) UV–vis absorption spectra of Bi12O17Cl2 nanomaterial.
D. Photocatalytic activity
Since the layer-stratiform structured Bi12O17Cl2 nanomaterial has a band gap as low as 2.1 eV and its visible-light absorption extends to 590 nm, it is expected that the Bi12O17Cl2 may exhibit superior visible-light-responsive photocatalytic performance. In photodegradation experiments, MO was adopted as the target pollutant. Figure 6 presents the variation of MO concentration with irradiation time under visible light. In the absence of Bi12O17Cl2 catalyst, photodegradation of MO under visible light is negligible, which suggests that the existence of Bi12O17Cl2 catalyst is the prerequisites for MO photodegradation. When adopting Bi12O17Cl2 as photocatalyst, MO concentration decreases promptly as visible-light irradiation time increases and the efficiency of MO decomposition is nearly 97% in 90 min, as demonstrated in Figure 6. The MO photodegradation experiments verify the as-synthesized Bi12O17Cl2 photocatalyst as an advanced visible-light-responsive photocatalyst.

Figure 6. (colour online) Photocatalytic activity and TOC decrement rate of Bi12O17Cl2 photocatalyst for MO degradation under visible-light irradiation.
The concentration of total organic carbon (TOC) was measured as a mineralization index to characterize MO degradation. As shown in Figure 6, 85% of TOC in the MO solution was eliminated after 90 min of visible-light irradiation in the presence of the Bi12O17Cl2 photocatalyst, indicating that most of MO was mineralized. The variation trend of TOC removal efficiency is consistent with that of photodegradation efficiency. In addition, the photostability of Bi12O17Cl2 photocatalyst was evaluated through recycling experiments for MO degradation under visible light, as shown in Figure 7. The catalyst was collected after degradation by filtration and reused for two times under the same conditions. Compared with the first run, there was gradual decline in degradation efficiency in the following two runs and the degradation rate was 86% in 90 min in the third run, which was 89% of that of the first run, showing that there is slight loss in activity for photocatalytic degradation of MO. The recycling experiments and TOC removal efficiency demonstrate that as-synthesized Bi12O17Cl2 is promising in future practical application.
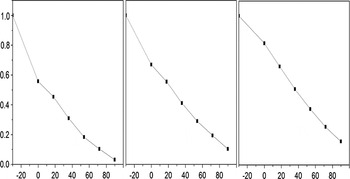
Figure 7. Recycling properties of Bi12O17Cl2 photocatalyst for MO degradation under visible-light irradiation.
E. Photocatalytic mechanism
During the dye photodegradation over semiconductor photocatalyst, there are three possible mechanisms: photolysis, dye photosensitization, and photocatalytic process. The photolysis process mainly involves the photoexcitation of dye molecules and the combination of electrons excited with O2 to produce single oxygen atoms, which serves as an oxidant for pollutant photodecomposition (Lin et al., Reference Lin, Huang, Huang, Wang and Shi2006). The photolysis process depends on the structure stability of dye molecules. As to the dye photosensitization process, there involves three steps: the dye molecules are photoexcited to induce electrons, electrons induced are injected to the conduction band of semiconductor photocatalysts, and electrons injected are captured by molecular oxygen to generate active species for dye photodegradation (Hu et al., Reference Hu, Xu and Zhao2004). For the photocatalytic mechanism (Li et al., Reference Li, Chen, Zhou and Shen2011a, Reference Li, Wang, Yao, Dang and Li2011b), semiconductor photocatalysts are directly excited and produce electrons and holes, as illustrated in Figure 8. A portion of the induced electrons and holes recombine in the surface of photocatalyst particles, the rest migrate to the surface of catalyst particles and react with dye molecules, subsequently, the photodegradation is performed. The photocatalytic process mainly lies on light absorption of semiconductor photocatalysts.

Figure 8. (colour online) Proposed direct photocatalytic mechanism over Bi12O17Cl2 under visible-light irradiation (CB: conducting band; VB: valence band).
On the basis of UV–vis diffuse reflectance spectrum, Bi12O17Cl2 photocatalyst could be excited by visible light to generate electrons and holes, which suggests that MO photodegradation under visible-light irradiation is dominated by direct photocatalytic mechanism. To provide additional information, a blank experiment (Figure 6) of MO degradation without any photocatalysts under visible light proves that MO is highly structural stable and MO decomposition by the photolysis mechanism can be neglected. For the role of photosensitization mechanism in MO degradation under visible light, Lin et al. studied the cut-off wavelength-dependent photocatalytic activity of TiO2 (Lin et al., Reference Lin, Huang, Huang, Wang and Shi2007). It was reported that the MO degradation over TiO2 at the cut-off wavelength of 450 nm is 4.0 and 86.5% under full arc. Since it is widely accepted that TiO2 cannot respond to visible light of λ > 450 nm, photosensitization mechanism plays the only role in the 4% portion of MO degradation and the portion of photosensitization process in the total MO degradation is about 4.6%. Therefore, the superior MO photodegradation performance of the as-synthesized Bi12O17Cl2 photocatalyst under visible-light irradiation is mainly attributed to the direct photocatalytic mechanism.
More significantly, the advanced photocatalytic performance is closely related with the morphology of Bi12O17Cl2 photocatalyst. As-synthesized Bi12O17Cl2 photocatalyst has a distinct hierarchical plate-stratiform nanostructure, which is composed of secondary nanosheets regularly arranged, with 10–50 nm in thickness and 200–1500 nm in in-plane size. Further, the secondary nanosheets are parallelly stacked by ultrathin primary nanosheets with the thickness in several nanometers. The contribution from the morphology and nanostructure to their photocatalytic activity is discussed as illustrated in Figure 8. Firstly, this plate-stratiform nanostructure has higher specific surface area, which could induce a much stronger dye adsorption capability on the surface of catalyst. From Figure 6, high adsorption capability of about 45% was achieved when the adsorption–desorption equilibrium of MO on catalyst surface was reached. Secondly, the plate-to-plate structure is believed to accelerate the transport of electrons and holes from catalyst to surface reaction sites and improve effective separation of photoinduced e–h pairs and thereby enhances the photocatalyst activity. Thirdly, the absorption edge of 590 nm is much larger than the data reported in the literature (Xiao et al., Reference Xiao, Liu, Hu, Zuo, Nan, Li and Wang2012, Reference Xiao, Jiang and Zhang2013), which leads to better light absorption in visible-light region. The increase of the absorption edge is attributed to the decrease of corresponding band gap energy value, which is very sensitive to the morphology and structure of photocatalysts. In this way, the Bi12O17Cl2 nanomaterial exhibits highly enhanced photocatalytic activity for the successful photodegradation of MO under visible-light irradiation.
IV. CONCLUSIONS
A novel plate-stratiform nanostructured Bi12O17Cl2 photocatalyst was synthesized, characterized, and adopted to photodegrade MO under visible-light illumination. The obtained Bi12O17Cl2 powder appeared as a stacked multilayer complex, which was composed of primary and secondary nanosheets. SAED and HRTEM results of a single Bi12O17Cl2 primary nanosheet reveal its highly single-crystalline nature. SAED and HRTEM analyses of a secondary nanosheet confirm the nature of plate-stacked microstructure. The absorption edge was determined as about 590 nm and the band gap energy was 2.1 eV. The photocatalytic activity of as-prepared Bi12O17Cl2 was studied by MO degradation under visible light and the degradation efficiency was nearly 97% in 90 min. 85% of TOC in the MO solution was eliminated after 90 min of visible-light irradiation, indicating that most of MO was mineralized. We assume that superior photodegradation performance is dominated by direct photocatalytic mechanism. The correlation between the microstructure and superior photocatalytic activity of Bi12O17Cl2 was studied. Our work may provide a prospect of adopting series of Bi-based oxychloride with beneficial layered structure as candidates for efficient and photo stable visible-light-driven photocatalysts.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0885715615000901
ACKNOWLEDGEMENTS
This work was partially supported by the International Science & Technology Cooperation Program of China (grant no. 2014DFA60150) and the National Natural Science Foundation of China (grant numbers 51172113 and 51373086). The authors thank Bei Wang and Shuning Li for their help in some experiments.









