I. INTRODUCTION
The title compound, 2-[((3R)-5-oxo-4-phenyltetrahydrofuran-3-yl)methyl] isoindoline-1,3-dione (PFIO), was obtained in the synthesis process of Milnacipran hydrochloride and reported for the first time. Milnacipran hydrochloride, 2-(aminomethyl)-N,N-diethyl-1-phenylcyclopropanecarboxamide hydrochloride, has been approved by FDA in January 2009, as an antidepressant of the serotonin–norepinephrine reuptake inhibitor (Naresh et al., Reference Naresh, Sait, Surendranath, Kaja and Kumar2012).
PFIO is an important impurity of Milnacipran hydrochloride. The successful synthesis, purification, and structure characterization gave a deep insight to the quality study of Milnacipran hydrochloride, and reactivity and reaction mechanism study of three-membered organic compounds. In this work, we synthesized PFIO and characterized the structural by melting point, ultraviolet spectrometry, element analysis, 1H-nuclear magnetic resonance (1H-NMR), single X-ray diffraction (SXRD), and X-ray powder diffraction (XRPD).
II. EXPERIMENTAL
A. Sample preparation and structure characterization
As shown in Figure 1, the title compound was obtained as a minor product (the ratio of PFIO and B is about 1:9) in the synthesis process of Milnacipran hydrochloride using a method described by Bernard et al. (Reference Bernard, Henri, Gilbert, Mike, Antoine, FranGois and Jean-Pierre1987). We proposed PFIO was formed in a way described in Figure 2. The purity of the title compound was tested by high-performance liquid chromatography. Its structure was characterized by the element analysis, ultraviolet spectrometry, 1H-NMR, and single-crystal diffraction. The melting point of the compound is 158–160 °C. The results of element analysis are consistent with the molecular formula (calculated values are C 71.02%, N 4.36%, H 4.71%, O 19.92%; and experimental values are C 70.97%, N 4.37%, H 4.38%), NMR data were collected as follows: 1H-NMR (400 MHz, CDCl3), δ (ppm):7.76 (s, 2H), 7.71 (s, 2H), 7.21 (s, 4H), 7.12 (s, 1H), 4.58 (t, J = 8.0 Hz, 1H), 4.15 (t, 1H), 4.01–3.96 (dd, J = 13.6,7.2 Hz, 1H), 3.88–0.383 (dd, J = 14.0, 6.0 Hz, 1H), 3.61 (d, 1H), 3.37–3.30 (m, 1H). The single-crystal sample of the title compound was obtained by dissolving the compound in dichloromethane and vaporing the solvent slowly. The single-crystal sample was ground into powder to collect the XRPD data. SXRD data were collected using an Oxford Diffraction Xcalibur Nova system with MoKα radiation (λ = 0.710 73 Å) at room temperature and the θ from 3.13° to 26.37°. The program package Olex2-1.1 (SHELX-97) was used for structure solution and refinement (Dolomanov et al., Reference Dolomanov, Bourhis, Gildea, Howard and Puschmann2009). The result of SXRD shows that the structure of the title compound was consistent with what we drawn and its crystal system is orthorhombic, space group is Pbca, and unit-cell parameters is a = 8.8615 (7), b = 14.6666(10), c = 24.4247(19) Å, α = β = γ = 90°, unit-cell volume V = 3174.4 Å3, and Z = 8. PFIO was arranged without intramolecular and intermolecular H bonding (see support information). Crystallographic data for PFIO were deposited with the Cambridge Crystallographic Data Center with a deposition number of CCDC-1404069.
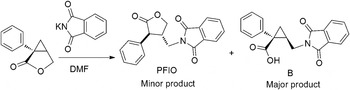
Figure 1. Structure and synthesis scheme of PFIO.

Figure 2. The formation mechanism of PFIO.
B) XRPD data collection and reduction
The diffraction pattern for the title compound was collected at room temperature using an X'Pert PRO diffractometer (PANalytical Co., Ltd., Netherlands) with an X'celerator detector and CuKα 1 radiation (λ = 1.540 56 Å, generator setting: 40 kV and 40 mA). The diffraction data were collected over the angular range from 4 to 50°2θ with a step size of 0.013 13°2θ and a counting time of 30 ms step−1. Data evaluation was performed using the software package Material Studio 4.2 (Accelrys Co., Ltd. USA).
The powder diffraction pattern was pretreated by subtracting the background and smoothing. Through analyzing the peak positions in the XRPD pattern by the DICVOL91 method from “Powder Indexing”, the preliminary unit-cell parameters were obtained. The indexing results were then refined using the Pawley refinement (Pan et al., Reference Pan, Guo, Duan, Cheng and Li2012), which involves assigning the Miller indices (h, k, l) to each observed peak in the experimental XRPD pattern (Harris, Reference Harris2012). After Pawley refinement, the final R wp of the spectrogram was converged to 8.56%. Powder Solve package (Engel et al., Reference Engel, Wilke, König, Harris and Leusen1999) was used to constantly adjust the conformation, position, and orientation of the trial model in a unit cell of PFIO. The result of Powder Solve was refined by Rietveld refinement techniques based on the experimental XRPD pattern. In the Rietveld refinement (Young, Reference Young1993), variables defining the structural model and the powder diffraction profiles were adjusted by least-squares methods for obtaining an optimal fit between the experimental pattern and calculated pattern. After the Rietveld refinement, the final R wp was 8.12%. The experimental powder diffraction pattern is depicted in Figure 3.
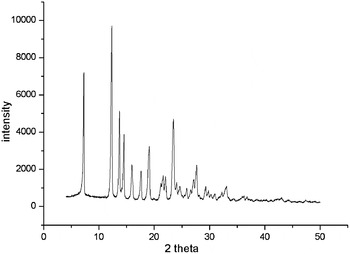
Figure 3. XRPD pattern of the PFIO, using CuK α1 radiation (λ = 1.540 56 Å).
III. RESULTS
The results of SXRD analysis show that PFIO is orthorhombic structure with space group Pbca and unit-cell parameters: a = 8.8615(7), b = 14.6666(10), c = 24.4247(19) Å, α = β = γ = 90°, unit-cell volume V = 3174.4 Å3, and Z = 8.
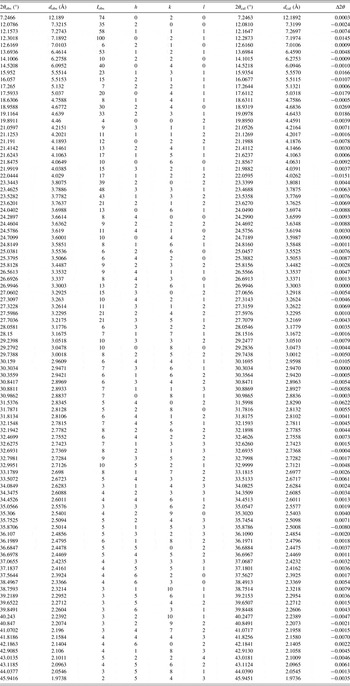
The experimental raw data were indexed by the DICVOL91 method. The figures of merit were achieved: F 22 = 24.1 (0.0132, 69) (Smith and Snyder, Reference Smith and Snyder1979) and M 22 = 10.6 (de Wolff, Reference de Wolff1968). The structure parameter calculated from powder diffraction data was consistent with SXRD analysis, as the orthorhombic structure with space group Pbca, and unit-cell parameters: a = 14.639(7), b = 24.378(3), c = 8.918(1) Å, α = β = γ = 90°, unit-cell volume V = 3182.7(9) Å3, and Z = 8. (The difference of lattice parameters directional principle for powder and single crystal makes the different sequence of abc of single crystal and powder.) The values of 2θ obs, d obs, I obs, h, k, l, 2θ cal, d cal, and Δ2θ are listed in Table I.
Table I. Indexed XRPD data of PFIO, C19H15NO4. Only the peaks with I rel of 1 or greater are reported [a = 14.639(7), b = 24.378(3), c = 8.918(1) Å, α = β = γ = 90°, unit-cell volume V = 3182.7(9) Å3, Z = 8, and space group Pbca]. All measured lines were indexed and are consistent with the Pbca space group. The d-values were calculated using CuKα 1 radiation (λ = 1.540 56 Å).
ACKNOWLEDGEMENTS
This work was supported by the Applied Basic Research Project of Sichuan Province (Grant no. 2014JY0042), the Testing Platform Construction of Technology Achievement Transform of Sichuan Province (Grant no. 13CGPT0049), and the National Development and Reform Commission and Education of China (Grant no. 2014BW011).
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at http://dx.doi.org/10.1017/S0885715616000105







