I. INTRODUCTION
The title compound drospirenone (Figure 1), systematic name 6,7,15,16-dimethylen-3-oxo-17-pregn-4-ene-2,17-carbo-lactone, is a novel progestogen with anti-mineralocorticoid and anti-androgenic characteristics (Muhn et al., Reference Muhn, Fuhrmann, Fritzemeier, Krattenmacher and Schillinger1995; Cagnacci et al., Reference Cagnacci, Piacenti, Zanin, Xholli and Tirelli2014). Drospirenone can counteract the estrogen-induced stimulation of the reninangiotensin–aldosterone system and blocks testosterone from binding to androgen receptors (Krattenmacher, Reference Krattenmacher2000). Hence, it has the potential to reduce body weight, blood pressure, and low-density lipoprotein levels and to enhance high-density lipoprotein levels.
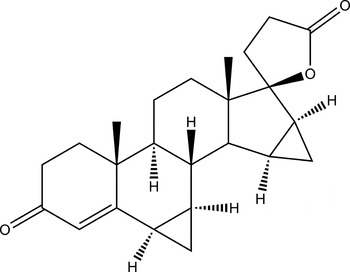
Figure 1. Structural formula of drospirenone.
The single crystallographic data of drospirenone [a = 12.236(2) Å, b = 12.592(2) Å, c = 12.879(2) Å, α = β = γ = 90°, unit-cell volume V = 1984.25 Å3, Z = 4, ρ cal = 1.227 g cm−3, and space group P212121] was obtained by Parisi et al. (Reference Parisi, Freire, Rusjan, Moreno, Bonadeo and Vega2013). To date, the detailed X-ray powder diffraction (XRD) data for drospirenone have not been reported.
II. EXPERIMENTAL
A. Sample preparation
The title compound was purchased from Zhejiang Xianju Pharmaceutical Co., Ltd., China (high-performance liquid chromatography, HPLC ≥ 98%) and characterized by ultraviolet and Fourier transform infrared spectrometer. The melting point and measured density of drospirenone are 200–201 °C and 1.236 g cm−3, respectively. Crystallization of drospirenone at room temperature was successful using ethyl acetate as solvent. Then, part of crystals was dried and ground into powder.
B. Diffraction data collection and reduction
XRD measurement was performed at room temperature using an X'Pert PRO diffractometer (PANalytical Co., Ltd., Netherlands) with a PIXcel one-dimensional detector and CuKα radiation (generator setting: 40 kV and 40 mA). The diffraction data were collected over the angular range from 5 to 50°2θ with a step size of 0.013 13°2θ and a counting time of 30 ms step−1.
The software package Material Studio 4.2 (Accelrys Co., Ltd., USA) was used to process the data in the State Key Laboratory of Polymer Materials Engineering (Sichuan University, China). The XRD pattern was pre-treated by subtracting the background, smoothing, and stripping off the Kα 2 component (Li et al., Reference Li, Wu, Pan, Cheng and Li2014). Automatic indexing results were obtained by the X-Cell method (Neumann, Reference Neumann2003; Wu et al., Reference Wu, Tang, Li, Zhang and Li2014). The indexing results were then refined using Pawley, which involves assigning the Miller indices (h, k, l) to each observed peak in the experimental powder XRD pattern (Wang et al., Reference Wang, Zhang, Wu, Pan, Su and Li2013).
C. Single-crystal XRD
XRD data for drospirenone were collected on an Xcalibur, Eos diffractometer. The crystal was kept at 293.15 K during data collection. The structure was solved with olex2 (Dolomanov et al., Reference Dolomanov, Bourhis, Gildea, Howard and Puschmann2009), a structure solution program using charge flipping and refined with the ShelXL (Sheldrick, Reference Sheldrick2008) refinement package using least-squares minimization.
III. RESULTS
Pawley refinement results confirmed that drospirenone is orthorhombic with space group P212121 and unit-cell parameters: a = 12.897(1) Å, b = 12.618(1) Å, c = 12.252(1) Å, α = β = γ = 90°, unit-cell volume V = 1994.13 Å3, Z = 4, and ρ cal = 1.229 g cm−3. The values of 2θ obs, d obs, I obs, h, k, l, 2θ cal, d cal, and Δ2θ are listed in Table I.
TABLE I. Indexed XRD data for drospirenone, C24H30O3. The d-values were calculated using CuKα 1 radiation (λ = 1.540 56 Å).

The single crystallographic data of drospirenone [a = 12.2379(5) Å, b = 12.6069(8) Å, c = 12.8921(8) Å, α = β = γ = 90°, unit-cell volume V = 1989.01(19) Å3, Z = 4, and ρ cal = 1.224 g cm−3] and the experimental data are listed in Table SI.
The comparison of XRD pattern with the simulated patterns of single-crystal structure and Parisi's is shown in Figure 2. Results showed that both single-crystal and powder diffraction methods can get similar structure data.
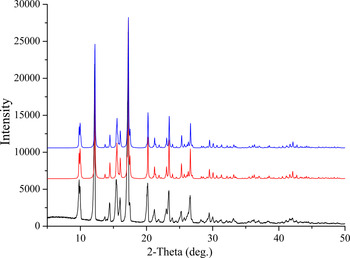
Figure 2. XRD pattern of drospirenone using CuKα radiation (black line) and the simulated pattern of single-crystal structure (red line) and Parisi's (blue line).
SUPPLEMENTARY MATERIALS AND METHODS
The supplementary material for this article can be found at http://dx.doi.org/10.1017/S0885715615000822






