Challenge of individual variability of response
There is a high degree of variability in treatment response among patients regardless of age (Fig. 1) (Holmes et al., Reference Holmes, Lazar, Melia, Astle, Dagi, Donahue, Frazier, Hertle, Repka, Quinn and Weise2011), suggesting that age is only one of many factors determining treatment response. Poor compliance with prescribed treatment has most often been blamed for a suboptimal treatment response, but when the actual patching time is measured (using occlusion dose monitors) (Fielder et al., Reference Fielder, Auld, Irwin, Cocker and Moseley1994), it is apparent that only a fraction of the variability can be explained by compliance (Stewart et al., Reference Stewart, Moseley, Stephens, Fielder and MOTAS Cooperative2004; Stewart et al., Reference Stewart, Stephens, Fielder, Moseley and ROTAS Cooperative2007). Since multiple factors (some known and many unknown) affect the amblyopia treatment response, clinicians are poorly equipped to make recommendations for amblyopia treatment (regarding whether to treat and how to treat) and currently have to approach each patient as an ‘average patient’.
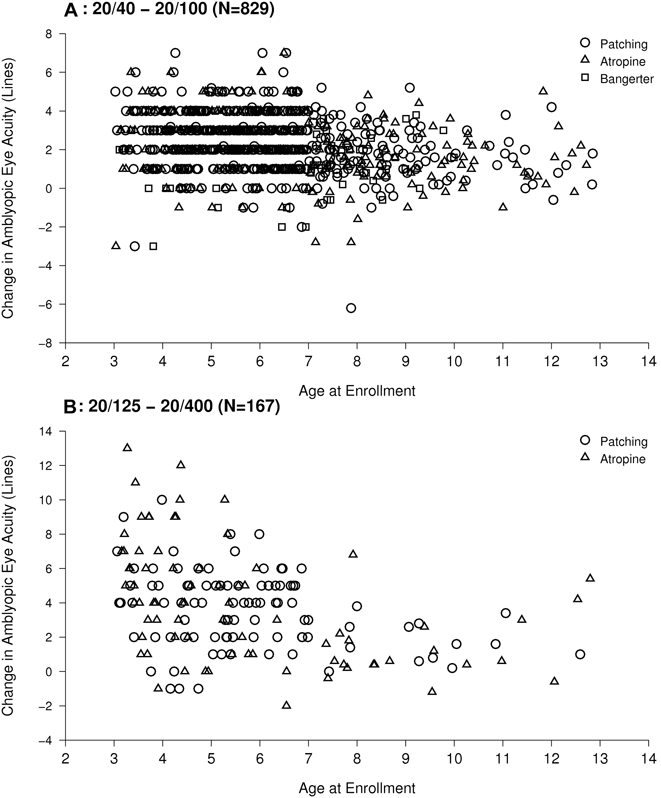
Fig. 1. Relationship between age and amblyopic eye visual acuity improvement, in children 3 to less than 13 years of age with moderate amblyopia (20/40 to 20/100, n = 829, A) or severe amblyopia (20/125 to 20/400, n = 167, B) from a meta-analysis of 4 amblyopia treatment trials (Pediatric Eye Disease Investigator Group, 2008a,b; Pediatric Eye Disease Investigator Group, 2009; Pediatric Eye Disease Investigator Group, 2010). Regarding improvement, starting at 20/40, it would take 3 lines improvement to reach 20/20, whereas starting at 20/200, it would take 10 lines improvement to reach 20/20 (used with permission).
Clearly, there is a pressing need to identify the many factors that influence the treatment response, of which age is only one.
Age considerations for current treatment modalities
Overall, there appears to be a reduction in the effect of common forms of treatment (patching, atropine, and Bangerter filter) with increasing age, particularly over the age of 7 years (Fig. 1). Stewart et al. (Reference Stewart, Moseley and Fielder2003) reported that comparing children <4 years, 4 to 6 years, and ≥6 years old, the outcome for all was similar, but for older children this required a greater dose of patching compared with those <4 years old.
Nevertheless, in many previous large randomized treatment trials of patching and atropine, ‘age’ has not been found to be an effect modifier, in that there appears to be no greater or lesser effect of patching vs. atropine, different doses of patching or different doses of atropine, dependent on age (Pediatric Eye Disease Investigator Group, 2002; Pediatric Eye Disease Investigator Group, 2003b; Pediatric Eye Disease Investigator Group, 2003c; Pediatric Eye Disease Investigator Group, 2004). Even when patching has been compared with continued optical treatment alone (after maximal improvement with optical treatment alone), age was not found to be an effect modifier (Pediatric Eye Disease Investigator Group, 2006a). The failure to find an age effect in these earlier PEDIG studies may have been due to the limitations of the study population (age 3 to <7 years), not including children >7 years old, where a modest effect of age begins to appear (Holmes et al., Reference Holmes, Lazar, Melia, Astle, Dagi, Donahue, Frazier, Hertle, Repka, Quinn and Weise2011).
Despite these findings, there are several practical and theoretical ways in which age may affect the outcome of specific modalities of the amblyopia therapy. For example, patient age may influence the ability and/or willingness to comply with a specific treatment. Some treatments, such as patching and atropine, may be easier for infants, who can less effectively resist treatment, whereas they may be resisted more strongly and more effectively by older children.
Recent studies have confirmed the importance of refractive correction (optical treatment) for amblyopia, regardless of whether the cause of the amblyopia is anisometropia, strabismus or both (Moseley et al., Reference Moseley, Neufeld, McCarry, Charnock, McNamara, Rice and Fielder2002; Moseley et al., Reference Moseley, Fielder and Stewart2009; Pediatric Eye Disease Investigator Group, 2006b; Pediatric Eye Disease Investigator Group, 2012). ‘Optical treatment of amblyopia’ can be defined as the long-term effect of putting a focused image on the retina of an amblyopic eye, in contrast to the immediate effect of correcting optical blur. The instantaneous improvement of visual acuity when correcting the blur with refractive correction would not be considered “treating” amblyopia, but the slow improvement of visual acuity over weeks and months while wearing new refractive correction would be considered ‘optical treatment’ of amblyopia. Perhaps, paradoxically, when also considering the effect of patching, atropine, and Bangerter filters (Fig. 1), the effectiveness of the optical treatment of amblyopia may be independent of age, with marked improvement in some teenagers (Pediatric Eye Disease Investigator Group, 2005) and even in adults (B. Thompson, personal communication). It would be worthwhile to formally study the effect of optical treatment of amblyopia in adults to substantiate these initial observations. From the standpoint of future clinical trial design, patients enrolled in an amblyopia treatment trial should first be provided with optimal refractive correction and have achieved maximal benefit from that optical treatment prior to the baseline visual acuity assessment and commencement of additional treatment. If optical treatment is not completed prior to starting the additional treatment to be studied, it will be impossible to separate the improvement due to optical treatment from the improvement from the treatment to be evaluated. It is also entirely possible that optical treatment of amblyopia and patching treatment of amblyopia might be mediated by entirely different neural mechanisms, and, therefore, it is important to separate these effects when designing future studies.
Correcting refractive errors even before amblyopia develops may be very important for preventing amblyopia. Atkinson’s research group (Anker et al., Reference Anker, Atkinson, Braddick, Nardini and Ehrlich2004; Atkinson et al., Reference Atkinson, Braddick, Nardini and Anker2007) reported that infants with hyperopia of at least +3.50 D (identified by photorefractive screening, confirmed on cycloplegic retinoscopy following screening) who wore spectacle correction were much less likely to have reduced visual acuity (including amblyopia) at age 4 years than those who did not. The Pediatric Eye Disease Investigator Group is currently conducting a similar study in 1–5 years olds, which may confirm or refute these findings, but further studies involving the identification of amblyogenic refractive errors by photoscreening and the prophylactic treatment of amblyogenic factors are needed. Some initial population-based work has been published on the concurrent association of specific refractive errors with amblyopia (Tarczy-Hornoch et al., Reference Tarczy-Hornoch, Varma, Cotter, McKean-Cowdin, Lin, Borchert, Torres, Wen, Azen, Tielsch, Friedman, Repka, Katz, Ibironke and Giordano2011), but longitudinal studies are needed to determine what levels of refractive error, if untreated, lead to amblyopia and in what proportion of children.
Patching of the nonamblyopic eye has been the cornerstone of amblyopia treatment for many years. Recent randomized clinical trials (RCTs) have substantiated the effectiveness of specific prescribed patching regimens for both moderate and severe anisometropic, strabismic, and combined-mechanism amblyopia (Pediatric Eye Disease Investigator Group, 2003a; Pediatric Eye Disease Investigator Group, 2003b). Lack of compliance remains a major pitfall in patching therapy (Stewart et al., Reference Stewart, Moseley, Stephens, Fielder and MOTAS Cooperative2004; Stewart et al., Reference Stewart, Stephens, Fielder, Moseley and ROTAS Cooperative2007) and may mediate some of the age effects of generally lesser improvement with increasing age (Holmes et al., Reference Holmes, Lazar, Melia, Astle, Dagi, Donahue, Frazier, Hertle, Repka, Quinn and Weise2011). For example, intensive patching regimens (e.g., >2 h daily) are much easier to implement in preschool children than in school-aged children because of different visual demands and psychosocial concerns (Pediatric Eye Disease Investigator Group, 2003c). Nevertheless, patching remains the standard for comparison for alternative and new amblyopia therapies.
Pharmacologic penalization (with atropine drops administered to the fellow eye) is also used to treat amblyopia and is supported by a series of RCTs demonstrating similar treatment effectiveness of daily or weekend atropine compared with part-time patching for anisometropic, strabismic, and combined amblyopia (Pediatric Eye Disease Investigator Group, 2002; Pediatric Eye Disease Investigator Group, 2004). Although applying an eye drop seems less onerous to some patients and parents compared with patching, the efficacy of penalization regimens may also be limited by poor compliance due to the child resisting the drop. There are some drawbacks associated with penalization. First, since the sound eye is blurred for the full day, school-aged children must rely on their amblyopic eye to do their schoolwork (unless prescribed a separate pair of glasses for reading). Second, in younger children (<3 years old), reverse amblyopia is difficult to detect because these young children often can rarely complete the optotype visual acuity testing and remains a concern with any continuous treatment.
Emerging treatment modalities
Several emerging therapies present potential advantages but also potential new drawbacks over patching and atropine in patients of specific ages with amblyopia. Shutter glasses/goggles transiently occlude the fellow eye at various frequencies either as an alternative method of occlusion (BenEzra et al., Reference BenEzra, Herzog, Cohen, Karshai and BenEzra2007) or to occlude alternate eyes in an effort to eliminate suppression (when alternating at high frequency). At low frequency, the shutter glasses might produce problems similar to those of patching: preventing activities such as school work in school-age children; however, in small pilot studies using 45 s on and 55 s off (BenEzra et al., Reference BenEzra, Herzog, Cohen, Karshai and BenEzra2007) and 30 s on and 30 s off (Wang et al., Reference Wang, Neely, Galli, Schleisser, Graves, Damarjian, Kovarik, Bowsher, Smith, Donaldson, Haider, Roberts, Sprunger and Plager2016), the shutter glasses were well tolerated.
New approaches, including perceptual learning and video game play (both monocular and dichoptic), seem to be promising additions to the amblyopia treatment armamentarium (Hess & Thompson, Reference Hess and Thompson2015; Vedamurthy et al., Reference Vedamurthy, Nahum, Huang, Zheng, Bayliss, Bavelier and Levi2015). Recent reviews, and meta-analyses of case series, suggest that visual acuity improvement with such treatments is modest, on average 0.1 to 0.2 logMAR, although other modalities of vision also improve (Levi, Reference Levi2012; Levi et al., Reference Levi, Knill and Bavelier2015; Tsirlin et al., Reference Tsirlin, Colpa, Goltz and Wong2015). It is important to note that for many of these studies, the treatment period was quite limited, typically 2 or 4 weeks. The current binocular (dichoptic) therapy of amblyopia is based on the principle of antisuppression therapy to promote simultaneous use of both eyes by decreasing the contrast and/or luminance of the fellow eye in order to equalize the perceptual strength of the input to the two eyes and to encourage fusion. Several game formats have been created, including ones that can be performed on a tablet or PC (Li et al., Reference Li, Thompson, Deng, Chan, Yu and Hess2013; Li et al., Reference Li, Jost, Morale, Stager, Dao, Stager and Birch2014). In children able to play the game and in adults, we would expect better compliance with such binocular games than with patching, (Kelly et al., Reference Kelly, Jost, Dao, Beauchamp, Leffler and Birch2016) although initial large randomized trials have been disappointing (Holmes et al., Reference Holmes, Mahn, Lazar, Beck, Birch, Kraker, Crouch, Erzurum, Khuddus, Summers and Wallace2016). Through the success and experience of the gaming industry, much is known about how to incentivize game play in children and young adults, and the same principles can be applied to optimize these therapeutic approaches for amblyopia. On the other hand, these games require dedicated time away from schoolwork or other activities that could otherwise be performed with a patch. Importantly, only children old enough to understand and interact with these devices and games can benefit from these therapies. Passive binocular activities, such as watching dichoptic movies (Li et al., Reference Li, Reynaud, Hess, Wang, Jost, Morale, De La Cruz, Dao, Stager and Birch2015; Bossi et al., Reference Bossi, Tailor, Anderson, Bex, Greenwood, Dahlmann-Noor and Dakin2017), may be a more practical approach for even younger children or patients with neuro-cognitive or other developmental impairment. A major limitation is providing specific games or movies that are sufficiently engaging. However, it may be possible to merge the passive approach with computer displays used for homework, reading, and entertainment, which would provide the variety that could support consistent treatment.
Regarding pharmacological approaches to treating amblyopia, although a recent RCT investigating patching with and without systemic oral levodopa had disappointing results (Pediatric Eye Disease Investigator Group, 2015), there is continued interest in other systemic pharmacologic therapies combined with conventional therapy (such as patching), predicated on the hypothesis that pharmacologic manipulation of the molecular ‘brakes’ that preclude synaptic plasticity will facilitate a more robust response to treatment. These pharmacologic approaches include selective serotonin reuptake inhibitors (Maya Vetencourt et al., Reference Maya Vetencourt, Sale, Viegi, Baroncelli, De Pasquale, O’Leary, Castren and Maffei2008), acetylcholine esterase inhibitors (Morishita et al., Reference Morishita, Miwa, Heintz and Hensch2010), and histone deacetylase inhibitors (Bavelier et al., Reference Bavelier, Levi, Li, Dan and Hensch2010; Silingardi et al., Reference Silingardi, Scali, Belluomini and Pizzorusso2010), and temporary binocular inactivation of retinal ganglion cells with the blockade of sodium channels (Fong et al., Reference Fong, Mitchell, Duffy and Bear2016). While these approaches may hold some promise, there are practical issues for consideration. First, some of these medications are psychoactive, having significant adverse effect profiles in adults that have not yet been studied in children, and they currently are only FDA-approved for unrelated use in adults. Second, systemic administration is likely to have effects throughout the central nervous system, not just in the visual cortex. Unintended effects on synaptic physiology elsewhere in the brain could have unforeseen effects on neurodevelopment which would need to be carefully evaluated before such medications could be used for treating amblyopia.
Similar considerations may apply to transcranial magnetic or direct current stimulation (Thompson et al., Reference Thompson, Mansouri, Koski and Hess2008) and complete darkness (He et al., Reference He, Ray, Dennis and Quinlan2007; Bavelier et al., Reference Bavelier, Levi, Li, Dan and Hensch2010; Duffy & Mitchell, Reference Duffy and Mitchell2013). In contrast to the established, and perhaps more benign, therapies (such as optical treatment, patching and atropine), much less is known about the unintended and adverse effects with these proposed new treatments. It would seem reasonable that the initial studies should first be conducted on adults, and perhaps on nonhuman primates before humans, avoiding many of the special ethical and regulatory issues pertaining to children. That said, a negative result in a study of adults with amblyopia should not necessarily be interpreted as a complete failure of the therapy, and studies in children with amblyopia may still be warranted with appropriate attention to safety, consent, and ethics.
Role of motor activity
Recent animal studies raise the question of whether motor activity could be relevant to the treatment of amblyopia. Is it possible that the treatment of amblyopia could benefit from concurrent motor activity? Experiments in rodents have suggested that motor activity in addition to the visual system stimulation promotes recovery from monocular deprivation. In adult rats reared in an enriched environment where they can run on wheels and have toys to play with in the company of other rats, the loss of visual acuity resulting from monocular deprivation is reversed (Sale et al., Reference Sale, Maya Vetencourt, Medini, Cenni, Baroncelli, De Pasquale and Maffei2007; Greifzu et al., Reference Greifzu, Pielecka-Fortuna, Kalogeraki, Krempler, Favaro, Schlüter and Löwel2014). When the various components of the environmental enrichment are isolated, enhanced physical exercise, enhanced visual enrichment, and perceptual learning are all shown to contribute to the recovery but social enrichment does not (Baroncelli et al., Reference Baroncelli, Bonaccorsi, Milanese, Bonifacino, Giribaldi, Manno, Cenni, Berardi, Bonanno, Maffei and Sale2012). Moreover, mice running on a styrofoam ball have rather more plasticity in the visual cortex than mice that are stationary. Evidence suggests that motor-related signals can gain access to the visual cortex via two different ‘neuromodulatory’ routes. One is carried by acetylcholinergic afferents from the basal forebrain to the parvalbumin cells, resulting in disinhibition of visual cortical circuits in the visual cortex (Stryker, Reference Stryker2014). The other involves a more direct excitatory input from the nuclei of the visual thalamus (including the lateral geniculate nucleus) that is probably inherited from the head and body movement-related activity in the superior colliculus (Roth et al., Reference Roth, Dahmen, Muir, Imhof, Martini and Hofer2016).
Held showed more than 50 years ago that plasticity in the visual system is affected by feedback from the motor system (Held, Reference Held1965). There are indications that the widespread augmentation of cholinergic activation of human sensory-motor and attention-based cortical systems can enhance perceptual learning of the novel, behaviorally-relevant visual tasks (Rokem & Silver, Reference Rokem and Silver2010). However, the degree to which the addition of the visuomotor feedback and motor activity to treatment may be useful is unresolved and would benefit from rigorous assessment. Investigation of whether the response to amblyopia therapy is enhanced by a motor or by a visuomotor input could begin to address this question. Appropriately randomized and controlled studies in humans would ultimately be required.
If concurrent motor activity is found to enhance plasticity, it should be explored for integration into the treatment of amblyopia, except perhaps in the very youngest infants where specific motor activity may be difficult to direct and control, or in patients who have other disabilities that preclude a specific type of motor activity. In addition, if general or specific motor activity is found to enhance improvement of amblyopic eye visual acuity, it may be useful when treating adults where, on average, response to other treatments may be somewhat more limited.
Areas of focus and ongoing challenges
As we consider future studies of new treatments for amblyopia and how patient age might influence their effectiveness, there are several challenges that merit further discussion. First and perhaps, most importantly, individual responses to all amblyopia treatments appear to be highly variable (Fig. 1). From the standpoint of clinical trial design, high variability drives up the needed sample size and, in turn, the cost of conducting a treatment trial. In addition, we need outcome measures beyond optotype visual acuity for amblyopia treatment trials. Novel biomarkers and better categorization of amblyopia may help address this problem of variability of the outcome. Regarding better categorization of amblyopia, there are practical limitations to increasing the number and complexity of tests used within a multicenter clinical trial design, and therefore additional focused ‘deep phenotyping’ studies are needed to help define a limited set of high-yield tests. Such ‘deep phenotyping’ projects will probably involve a small number of interested dedicated sites.
Compliance remains an ongoing challenge. When combined with prescribed patching, educational regimens have been found to improve treatment adherence (Loudon et al., Reference Loudon, Fronius, Looman, Awan, Simonsz, van der Maas and Simonsz2006). Measures of actual duration of the game play can also now be incorporated into electronic games that are being developed for amblyopia treatment, as a more direct assessment of compliance. Incorporating objective measures of compliance, such as occlusion dose monitors (Fielder et al., Reference Fielder, Auld, Irwin, Cocker and Moseley1994) for patching and actual measure of the treatment duration for hand-held or computer-based treatment, would be beneficial in future amblyopia treatment trials.
The choice of outcome measures for future amblyopia treatment trials also deserves re-evaluation. The current standard outcome measure of optotype visual acuity has proven to have a high degree of test-retest variability (Holmes et al., Reference Holmes, Beck, Repka, Leske, Kraker, Blair, Moke, Birch, Saunders, Hertle, Quinn, Simons and Miller2001; Beck et al., Reference Beck, Moke, Turpin, Ferris, SanGiovanni, Johnson, Birch, Chandler, Cox, Blair and Kraker2003; Cotter et al., Reference Cotter, Chu, Chandler, Beck, Holmes, Rice, Hertle and Moke2003) and may not be the optimum or only important outcome measure for the amblyopia treatment trial. Contrast sensitivity (CS) is also impaired in amblyopia (Hess & Howell, Reference Hess and Howell1977; Levi & Harwerth, Reference Levi and Harwerth1977), but we need new methods for rapid evaluation of CS thresholds before we could use CS as a primary outcome measure for treatment trials. In addition, ongoing work (Sharma et al., Reference Sharma, Levi and Klein2000; Popple & Levi, Reference Popple and Levi2008; Hou et al., Reference Hou, Kim, Lai and Verghese2016) has highlighted the importance of higher order visual processing in amblyopia, and we need methods to efficiently assess those parameters in a clinical setting. Recent emphasis on treatment using dichoptic tasks suggests that measurement of suppression and binocular fusion may be especially important. There is an opportunity for new visuo-motor tests to be incorporated into the software used on the new therapeutic devices (such as hand held tablets) which may lower the burden of testing these parameters in future clinical trials. Such clinical trials should focus not just on children with amblyopia but also on adults, who have hitherto been neglected in RCTs directed at amblyopia treatment.
Lastly, current measurements of visual function in the clinician’s office do not necessarily reflect the functional consequences of amblyopia and its treatment and their effects on health-related quality of life or the economic consequences of the condition and treatment. New patient-derived instruments to assess functional vision in children, and health-related quality of life in children and their parents, are under development (Liebermann et al., Reference Liebermann, Leske, Castañeda, Hatt, Wernimont, Cheng, Birch and Holmes2016; Hatt et al., Reference Hatt, Leske, Wernimont, Birch and Holmes2017). Appropriately designed, patient derived, questionnaire instruments can be used in both clinical and research environments without placing a significant burden on patient and families. Such questionnaires can easily be incorporated into amblyopia treatment trials because they can be completed by the patient and/or parents while waiting before and between clinical tests.
Recommendations
While amblyopia is a disorder rooted in the principles of critical periods for synaptic plasticity (Lewis & Maurer, Reference Lewis and Maurer2005), the concept of an ‘age limit’ for plasticity is being challenged, both in its occurrence and through pharmacologic manipulation. No longer viewing age as a relative contraindication for amblyopia treatment now should guide what treatments might be most appropriate and most likely to result in better compliance (adherence) and outcomes.
• Variability of response may not be so much driven by age and/or compliance but other, as yet unknown, factors that require further studies for elucidation. Such studies will probably involve ‘deep phenotyping,’ using new and existing clinical tests (beyond optotype visual acuity), and lead to an improved clinical classification scheme for amblyopia based on new and existing biomarkers. Such biomarkers may better predict the treatment response to current therapies such as patching and ultimately may guide decision-making and therapeutic approaches. New outcome measures with less variability may also allow more efficient treatment trials.
• New and evolving treatments show promise in therapeutic efficacy, particularly for adults, but we must consider the risks and adverse effects if considering these treatments for children.
• The impact of amblyopia on the patients’ functional vision and health-related quality of life, including the health-related quality of life of the parents and care-givers (in childhood amblyopia), along with economic consequences, should be a focus of future research.
Acknowledgments
The authors would like to thank Eric Gaier,* who served as scribe for this discussion section. We would also like to thank all discussion group participants: Jan Atkinson, Peter Bex, Eileen Birch, Susan Cotter, Alistair Fielder, David Hunter, Sjoukje Loudon, Al Sommer, Ben Thompson, and Larry Tychsen.
*Boston Children’s Hospital and Harvard Medical School, Boston, MA, USA.



