Triclopyr is a synthetic auxin herbicide in the pyridine carboxylic acid family that is used to control woody and herbaceous broadleaf plants in many settings (Shaner Reference Shaner2014). It was first registered in 1979 as a triethylamine salt formulation for forestry and non-crop use (EPA 1998). In 1980, a butoxyethyl ester formulation was registered for use in the same settings (EPA 1998). These amine and ester formulations have been widely used for more than 35 yr and have been subsequently labeled for several crops and non-crop uses, including aquatics, pastures, rice, rangelands, and turf sites (Anonymous 2016a, 2016b, 2016c, 2016d). Recently, two additional triclopyr formulations have been registered that include a pyridinyloxyacetic acid and a choline salt. Though these four triclopyr formulations are assumed to have a relatively similar weed control spectrum (Anonymous 2016a, 2016b, 2016c, 2016d), research directly comparing them has not been fully documented. This is especially true for the acid and choline formulations, for which little information exists. Given that they differ in other aspects such as cuticle permeability, label signal word, application sites, water solubility, volatility, and soil adsorptive capacity (Anonymous 2016a, 2016b, 2016c, 2016d; Shaner Reference Shaner2014), it is highly likely there is some differential performance among formulations.
Differential performance between pyridine carboxylic herbicides such as picloram and clopyralid has already been well established, but it is not an absolute. For example, Bovey et al. (Reference Bovey, Morton, Meyer, Flynt and Riley1972) found that the potassium salt formulation of picloram provided better control of yaupon (Ilex vomitoria Ait.) than the isooctyl ester formulation in hot, dry conditions in Texas. However, Bovey et al. (Reference Bovey, Ketchersid and Merkle1970) found similar or slightly higher activity with an isooctyl ester than the potassium salt formulation of picloram for woody plant control in tropical conditions in Puerto Rico. Kloppenburg and Hall (Reference Kloppenburg and Hall1990b) found greater absorption of an acid formulation of clopyralid in Canada thistle [Cirsium arvense (L.) Scop.] and wild buckwheat (Polygonum convolvulus L.) than other salt and ester formulations. This translated into greater efficacy for the acid formulation compared with the ethylhexyl ester formulation in additional growth chamber studies (Kloppenburg and Hall Reference Kloppenburg and Hall1990a). However, for triclopyr, Harrington and Miller (Reference Harrington and Miller2005) found no difference between amine and ester formulations for Chinese privet (Ligustrum sinense Lour.) control. Similarly, research by Hutchinson et al. (Reference Hutchinson, Langeland and Meisenberg2011) revealed that both the amine and ester formulations of triclopyr reduced hen’s eyes (Ardisia crenata Sims) cover by greater than 90% at 12 mo after treatment with no difference between the two.
Given that there are no known published studies comparing the triclopyr acid or choline salt formulations with the historic commercial standards, it is important to examine the effects of the various formulated products on the relative activity of triclopyr. This is especially true where significant confusion can occur among land managers over which triclopyr formulation should be used (SFE, unpublished data). To begin to address this issue, we conducted greenhouse dose–response bioassay studies with the four commercially formulated triclopyr products on four susceptible broadleaf crop species. We chose soybean, tomato, sunflower, and cotton as model species representing four key plant families (Fabaceae, Solanaceae, Asteraceae, and Malvaceae) that contain a large number of weedy species with varying degrees of susceptibility to triclopyr (Ferrell et al. Reference Ferrell, Mullahey, Langeland and Kline2006; Macdonald et al. Reference Macdonald, Brecke, Colvin and Shilling1994; Norsworthy et al. Reference Norsworthy, Bangarwa, Scott, Still and Griffith2010; Sellers and Ferrell Reference Sellers and Ferrell2017). If differential efficacy between triclopyr formulations is verified within greenhouse studies on these crop species that possess uniform germination, emergence, and growth rates, then additional field studies across a range of weedy species and environmental conditions would be highly justified.
Materials and Methods
Greenhouse dose–response experiments were conducted at the University of Florida in Gainesville in the fall of 2015. Soybean (‘Liberty-Link CZ 5515LL,’ Bayer Crop Science, Research Triangle Park, NC), tomato (‘Better Boy,’ Burpee, Warminster, PA), sunflower (‘Perodovik,’ Mixon Seed, Orangeburg, SC), and cotton (‘Phytogen 499 WRF,’ Dow AgroSciences, Indianapolis, IN) were selected for the study. Seeds from each plant species were planted into 5 by 5 cm pots uniformly filled with commercial potting medium (Fafard Mixes for Professional Use, Conrad Fafard, Agawan, MA) amended with 14–14–14 slow-release fertilizer (Osmocote Smart-Release Plant Food, Scotts-Sierra Horticultural Products, Marysville, OH). The experiment was conducted twice with plantings on October 1 and November 2. Pots were watered as needed, and the greenhouse temperature was maintained at 30/24 C day/night under a 14-h photoperiod using natural and supplemental lighting. Approximately 7 d after emergence, plants were thinned to 1 plant pot−1.
Triethylamine salt of triclopyr (Garlon® 3A, 357 gaeL−1, Dow AgroSciences, Indianapolis, IN), butoxyethyl ester of triclopyr (Remedy® Ultra, 476 gaeL−1, Dow AgroSciences, Indianapolis, IN), pyridinyloxyacetic acid of triclopyr (Trycera®, 342 gaeL−1, Helena Chemical, Collierville, TN), and choline salt of triclopyr (Vastlan™, 476 gaeL−1, Dow AgroSciences, Indianapolis, IN) were applied at rates of 17, 34, 67, 140, 280, 561, and 1,121 g ae ha−1. Applications were made using a compressed air–powered moving-nozzle spray chamber (Generation II Spray Booth, Devries Manufacturing, Hollandale, MN) equipped with a TeeJet® 8001 EVS spray nozzle (TeeJet Technologies Southeast, Tifton, GA) calibrated to deliver 187 L ha−1 at 172 kPa. All treatments and the nontreated control included a nonionic surfactant (Activator 90, Loveland Products, Greeley, CO) at 0.25% v/v.
Treatments were applied approximately 20 d after planting (DAP) to plants in the 2- to 3-leaf growth stage. Plants were returned to the greenhouse following application and maintained as previously described. The experiment was set up as a factorial of triclopyr formulation and triclopyr rate arranged in a completely randomized design with four replications for each species; a single pot served as the experimental unit.
Data Collection and Analyses
Aboveground fresh biomass (AFB) was harvested at the soil surface of each pot 20 d after treatment (DAT) and weighed. Data were subjected to ANOVA to test significance of main effects and interactions (α=0.05) (Pinheiro and Bates Reference Pinheiro and Bates2000). Nonlinear regression analysis was then performed for each species using the ‘drc’ package of R Studio v. 3.3.0 (Ritz and Streibig Reference Ritz and Streibig2005). A three-parameter log-logistic equation, similar to that proposed by Seefeldt et al. (Reference Seefeldt, Jensen and Fuerst1995), was fit to AFB percent-reduction data for each species using the regression model in Equation 1,
where Y is the response (AFB) expressed as a percent reduction compared with the nontreated control, x is triclopyr rate, b is the relative slope at the inflection point, d is the upper limit of the curve, and e is the inflection point of the fitted line (equivalent to the dose required to cause 50% response [ED50]). A lack-of-fit test at the 95% level comparing the regression model with ANOVA was conducted to determine whether the regression model was an appropriate fit to the data (Ritz and Streibig Reference Ritz and Streibig2005). Differences among parameter estimates (ED50) were compared using the Sidak multiple-comparison adjustment (α=0.0085) (Abdi Reference Abdi2007).
Results and Discussion
Treatment by run interactions were not significant for all species tested; therefore, data were pooled over experimental runs. A lack-of-fit test at the 95% level was not significant for all curves, indicating that the regression models were appropriate (Ritz and Streibig Reference Ritz and Streibig2005).
Within three of the four species tested, differences in activity were observed between triclopyr formulated products. Additionally, the pattern of triclopyr activity by formulation across species was not consistent. For soybean, the triclopyr amine and choline exhibited greater activity than the triclopyr ester or acid, as indicated by ED50 values that were approximately 50% lower (Table 1; Figure 1). The triclopyr ester and acid were not different from each other, as indicated by their ED50 values (Table 1; Figure 1). The inflection point slope (b) for triclopyr ester was higher than for triclopyr choline, suggesting that soybeans were more sensitive to increases in triclopyr ester rate than triclopyr choline rate near their respective ED50 values (Table 1). This behavior may be related to the higher initial absorption of ester formulations (Hess et al. Reference Hess, Bayer and Falk1981; Price Reference Price1983; Ozair et al. Reference Ozair, Moshier and Werner1987). In general, acid and ester formulations are considered to be less polar than salts and thus are rapidly absorbed; on the other hand, salts are highly polar, which results in reduced absorption (Ashton and Crafts Reference Ashton and Crafts1981; Currier and Dybing Reference Currier and Dybing1959; Loos Reference Loos1975).
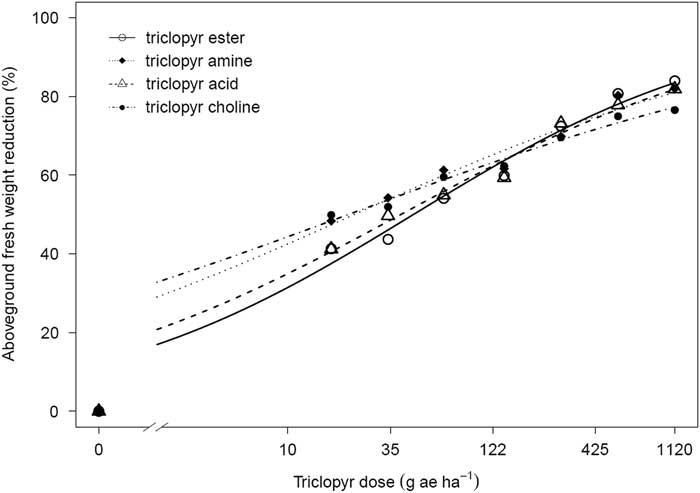
Figure 1 Aboveground fresh weight reduction of soybean in response to four triclopyr formulations 20 d after treatment. Triclopyr ester, amine, acid, and choline were applied at 0, 17, 34, 67, 140, 280, 561, and 1,121 g ae ha−1. A three-parameter logistic regression model of Y=d/1+exp[b(log x−log e)] was used to fit the data.
Table 1 Parameter estimates (±SE) in soybean, tomato, sunflower, and cotton with the use of a log-logistic model.Footnote a

a Log-logistic model: Y=d /1+exp[b(log x−log e)], where Y is the response (% aboveground fresh biomass reduction), x is the triclopyr rate, b is the slope of the inflection point, d is the upper limit of the curves, and e is the inflection point of the fitted line (equivalent to the dose [g ae ha−1] required to cause 50% response [ED50]).
b ED50 estimates followed by the same letter are not different according to the Sidak multiple-comparison adjustment (P≤0.0085).
Triclopyr amine resulted in the lowest ED50 value of 22.87 on tomato, which was approximately 40% lower than for triclopyr ester or choline and 50% lower than for triclopyr acid (Table 1; Figure 2). The ester, choline, and acid formulated products performed similarly on tomato as indicated by their ED50 values (Table 1). The pattern was somewhat similar for sunflower, as triclopyr amine had the lowest ED50 value of 60.39, which was approximately 23% lower than for the other three formulated products (Table 1; Figure 3). There were no differences among ester, choline, and acid formulated products on sunflower (Table 1). Despite the similar statistical results between tomato and sunflower, the differences among the formulated products were not as dramatic in sunflower as they were with tomato (Table 1; Figure 3).
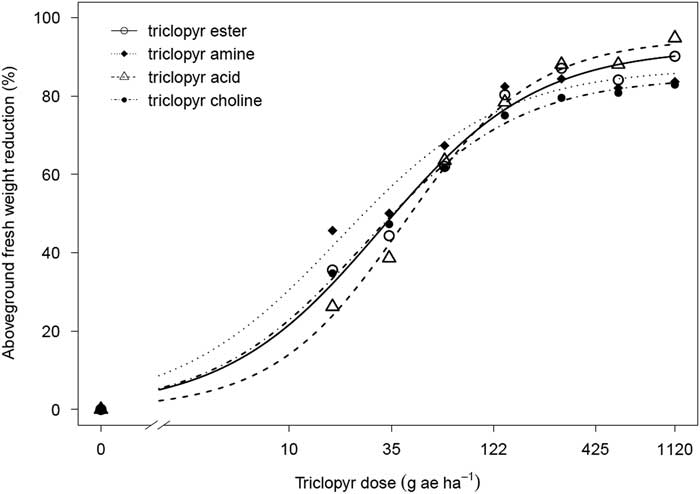
Figure 2 Aboveground fresh weight reduction of tomato in response to four triclopyr formulations 20 d after treatment. Triclopyr ester, amine, acid, and choline were applied at 0, 17, 34, 67, 140, 280, 561, and 1,121 g ae ha−1. A three-parameter logistic regression model of Y=d/1+exp[b(log x−log e)] was used to fit the data.

Figure 3 Aboveground fresh weight reduction of sunflower in response to four triclopyr formulations 20 d after treatment. Triclopyr ester, amine, acid, and choline were applied at 0, 17, 34, 67, 140, 280, 561, and 1,121 g ae ha−1. A three-parameter logistic regression model of Y=d/1+exp[b(log x−log e)] was used to fit the data.
No differences between ED50 values were observed for cotton (Table 1; Figure 4), as this species could be relatively more tolerant than the other three species to triclopyr (Bauerle et al. Reference Bauerle, Griffin, Alford, Curry and Kenty2015; Sciumbato et al. Reference Sciumbato, Chandler, Senseman, Bovey and Smith2004). In addition, a high level of variance was present for cotton ED50 values (average SE=9.4) compared with the other species. Another possible reason for the similarity among formulated products in cotton may be growth stage. Snipes et al. (Reference Snipes, Street and Mueller1991) determined that injury and yield reduction of cotton following applications of triclopyr at simulated drift rates (0 to 60 g ae ha−1) depended more on time of application than on triclopyr rate, as greater yield reductions were observed at early bloom than at the pinhead square stage.

Figure 4 Aboveground fresh weight reduction of cotton in response to four triclopyr formulations 20 d after treatment. Triclopyr ester, amine, acid, and choline were applied at 0, 17, 34, 67, 140, 280, 561, and 1,121 g ae ha−1. A three-parameter logistic regression model of Y=d/1+exp[b(log x−log e)] was used to fit the data.
Although we did not conduct 14C absorption and translocation studies as part of this work, the relative lower activity observed for the ester, acid, and choline formulated products in tomato and sunflower and the relative lower activity observed for the ester and acid formulated products in soybean may be due to differential absorption and translocation. Esters are generally absorbed at higher rates than acids due to their higher lipophilicity. However, the de-esterification process is the main limiting factor for translocation of ester formulations, because translocation of salt and acid formulations across the plasma membrane is not enzyme dependent (Bell et al. Reference Bell, Burke and Prather2011; Haslam et al. Reference Haslam, Raveton, Cole, Pallet and Coleman2001). This may explain the lower activity observed for the ester formulation in this study. These findings tend to agree with those of Kloppenburg and Hall (Reference Kloppenburg and Hall1990b). These authors compared, under controlled environment conditions, the phytotoxicity of five different clopyralid formulated products—the acid, monoethanolamine salt, potassium salt, 2-ethylhexyl ester, and 1-decyl ester—all applied to Canada thistle and wild buckwheat at the 8- to 10-leaf and 4- to 6-leaf stages of development, respectively. Analyzing shoot and root dry weight data from both species, they found that the 2-ethylhexyl ester was consistently less phytotoxic than the other four formulations, as we observed in this study.
In addition, the similar performance of the different triclopyr formulations may have also been due to the young growth stage of the plant species at the time of application, as well as the optimal growing conditions provided by the greenhouse. It is possible that the small size of the plants and the rapid growth conditions led to a much-reduced cuticle that did not serve as a barrier to herbicide entry. Schonherr and Baur (Reference Schonherr and Baur1994) suggested that both growth stage and environmental conditions can impact the wax content on the leaves and, consequently, herbicide absorption. Our experiment was conducted in a greenhouse setting, which does not promote thick cuticle wax growth (Hatterman-Valenti et al. Reference Hatterman-Valenti, Pitty and Owen2006); if a thicker cuticle was present in the current study, it is possible that more differences would have been detected among formulated products. These formulated products might respond differently if applied to more mature plants under field conditions. Furthermore, the effects of formulation on herbicide entry into the cuticle membrane may not be uniform across all plant species, and each plant/herbicide combination must be considered separately (Devine et al. Reference Devine, Duke and Fedtke1993).
In summary, these data suggest that triclopyr formulation can impact the degree of foliar activity in three (soybean, tomato, and sunflower) of the four species tested under greenhouse conditions. The amine formulation of triclopyr exhibited more growth reduction with tomato and sunflower than other formulations tested. In soybean, amine and choline salt formulations were similar to each other and more active than ester or acid formulations. Cotton did not respond differently to any of the formulations tested and was more tolerant than the other species. These results strongly support the need for additional research to compare the newer triclopyr acid and choline salt to the older amine salt and ester formulations. It is very likely that there may be considerable species-dependent activity that is highly influenced by environmental conditions. Clarifying these issues would greatly assist applicators in selecting the most appropriate triclopyr formulations for weed control operations. In addition, other important management factors such as applicator safety, volatility potential, and cost should be considered when choosing the best formulation to be applied.
Acknowledgments
The authors express their appreciation to Dennis C. Odero for assistance with the statistical analyses.







