INTRODUCTION
Understanding factors that shape species diversity is a long-standing goal in tropical ecology (Thornton et al. Reference THORNTON, BRANCH and SUNQUIST2011). With widespread forest fragmentation (Skole & Tucker Reference SKOLE and TUCKER1993), incorporating anthropogenic factors with more traditional biogeographic factors may better reveal the forces driving diversity. Information on how fragments support apex predators, such as larger owls, is of particular importance based on their role in regulating ecosystem processes (Magrach et al. Reference MAGRACH, LAURANCE, LARRINAGA and SANTAMARIA2014, Motta-Junior Reference MOTTA-JUNIOR2006).
Avian diversity in forest fragments is commonly affected by interactions among factors such as forest type and structure, surrounding land use, and fragment size and distance to larger tracts of primary forest (Durães et al. Reference DURÃES, CARRASCO, SMITH and KARUBIAN2013, Ribon et al. Reference RIBON, SIMON and DE MATTOS2003, Sberze et al. Reference SBERZE, COHN-HAFT and FERRAZ2010). These factors can be placed into two classes: fine-scale habitat variation (e.g. habitat structure, available light) and broader-scale fragment attributes (e.g. fragment area, surrounding tree cover). While many studies have evaluated forest fragmentation effects on diurnal bird species (Durães et al. Reference DURÃES, CARRASCO, SMITH and KARUBIAN2013, Gray et al. Reference GRAY, BALDAUF, MAYHEW and HILL2007, Sigel et al. Reference SIGEL, SHERRY and YOUNG2006), the relative importance of fine-scale vs. broader-scale attributes in shaping nocturnal bird communities remains poorly resolved.
In the Neotropics, nocturnal birds exhibit a wide range of habitat requirements, many of which are poorly understood (Enríquez et al. Reference ENRÍQUEZ, EISERMANN and MIKKOLA2012, Freile et al. Reference FREILE, GUEVARA, PACHECO and SANTANDER2015). In forest fragments, fine-scale habitat variables related to canopy disturbance can influence occurrence (Esclarski & Cintra Reference ESCLARSKI and CINTRA2014, Motta-Junior Reference MOTTA-JUNIOR2006, Ortiz-Pulido & Lara Reference ORTIZ-PULIDO and LARA2014). For example, open areas and increased light conditions may facilitate hunting and/or increase prey densities in the forest understorey (Lambert et al. Reference LAMBERT, MALCOM and ZIMMERMAN2006, Sekercioglu Reference SEKERCIOGLU2010). Fragment attributes at broader spatial scales are also likely influential in shaping patterns of diversity among nocturnal birds (Ibarra et al. Reference IBARRA, MARTIN, ALTAMIRANO, VARGAS and BONACIC2014). Within the Ecuadorian Andes, species richness generally increases with decreasing altitude (Freile et al. Reference FREILE, CASTRO and VARELA2012). In regard to fragment size, there was no influence on owl occurrence in forest fragments ranging from 10 to 180 ha in one study in Brazil (Kanegae et al. Reference KANEGAE, CAMACHO, FERNANDES, DOS SANTOS HONORATO, DE SOUZA FILHO and VIEIRA2012). Relationships between nocturnal bird diversity within fragments and surrounding tree cover has not been quantified (Sberze et al. Reference SBERZE, COHN-HAFT and FERRAZ2010).
To better understand how nocturnal bird species richness and community composition vary in relation to fine- and broad-scale ecological parameters, we surveyed 22 forest fragments in north-western Ecuador, a poorly sampled region where 11 species of nocturnal bird are currently experiencing population declines (Estrada & Boyla Reference ESTRADA and BOYLA2005, Freile & Castro Reference FREILE and CASTRO2013). We predicted richness would increase with decreasing altitude, greater canopy openness, increased fragment size and increased surrounding forest cover, and that community composition would vary along these same environmental gradients.
METHODS
Study area
From August to December 2014, we surveyed nocturnal avifauna in 22 forest fragments in and around the Mache-Chindul Ecological Reserve in north-west Ecuador (0o47′N, 79o78′W; Figure 1). This region corresponds to the southern extent of the Chocó biogeographic zone, an area with high biodiversity, endemism and threat (Orme et al. Reference ORME, DAVIES, BURGESS, EIGENBROD, PICKUP, OLSON, WEBSTER, DING, RASMUSSEN, RIDGELY, STATTERSFIELD, BENNETT, BLACKBURN, GASTON and OWENS2005). Intensive deforestation began in the 1960s in this area and continues to the present. Our sampling coincided with the dry season when many bird species breed in the region (Carrasco et al. Reference CARRASCO, BERG, LITZ, COOK and KARUBIAN2013). Fragments were comprised of a mix of primary and secondary tropical rain forest surrounded by cleared forestland under agricultural use, and varied in size from 2.7 to 33.6 ha (mean ± SD = 16.0 ± 9.6 ha). Altitude ranged from 135 to 592 m asl (mean ± SD = 345 ± 154 m asl) across all sites.
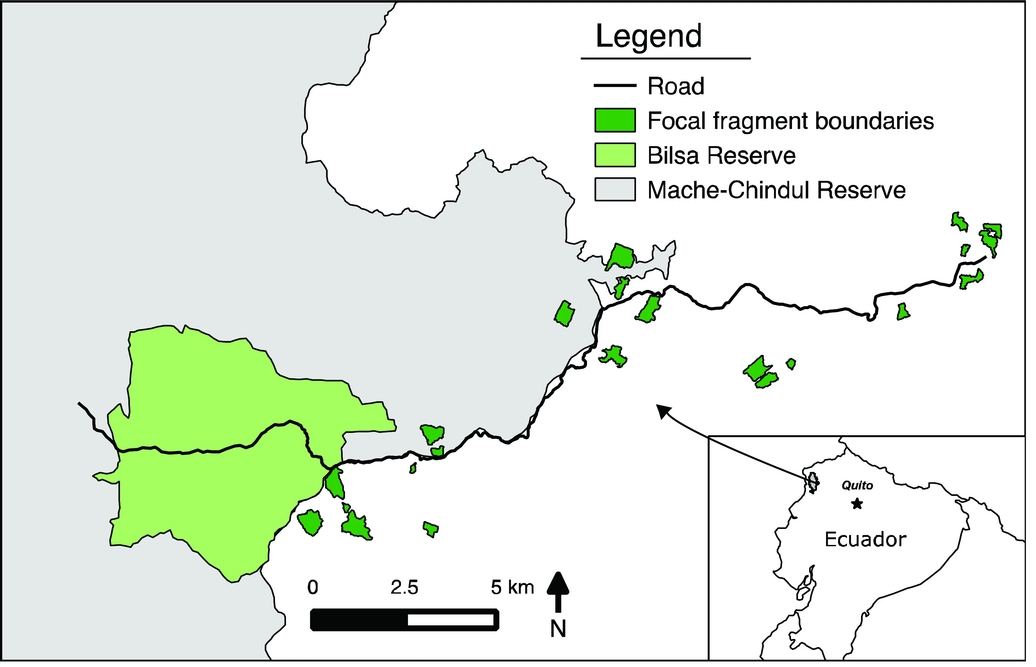
Figure 1. Map of study area and forest fragments surveyed for nocturnal birds in north-west Ecuador from August 2014 to December 2014. We analysed data from 22 focal fragments, depicted in dark green. Inset shows location of Mache-Chindul Reserve within Ecuador. This map only shows forest fragments sampled in this study; there are many additional fragments that occur within the region that are not depicted.
Field data
After manually mapping the border of each fragment with a hand-held GPS unit (Garmin), we established 500-m linear survey transects that began at the edge of each fragment and ran toward and through the fragment centre. Transects longer than 500 m would have often extended beyond the borders of our smaller fragments, and sampling the outside matrix was beyond the scope of the current study. In larger fragments, the transect bisected the fragment whereas in smaller fragments it angled back and forth in order to accommodate the full length of the transect. Transects did not overlap each other. Nocturnal birds within the region included owls, nightjars and potoos (Carrasco et al. Reference CARRASCO, BERG, LITZ, COOK and KARUBIAN2013, Table 1) and surveyors were trained in their auditory identification, by songs and calls, using recordings of all species that may occur in the region (Moore et al. Reference MOORE, KRABBE and JAHN2013).
Table 1. Names, numbers of detections, and numbers of forest fragments in which nocturnal bird species (Ridgely & Greenfield Reference RIDGELY and GREENFIELD2006) were detected during nocturnal surveys across 22 fragments in north-west Ecuador from August to December 2014. Numbers of detections are the quantity of surveys (out of 264) when the species was detected at least once.

During study nights, four separate surveys were conducted within a given fragment by two or more observers slowly walking the transect length and stopping every 50 m to listen for >3 min. Two surveys were conducted in the period between 18h36 and 23h35 UTC (average start time = 20h03). Surveyors began the first survey at 0 m on the transect (i.e. the fragment's edge) and walked to 500 m, at which time they waited at least 15 min before backtracking up the transect for the second survey of the night. Two additional surveys were conducted during the period between 0h00 and 6h45 (average start time = 4h29) by again walking down and back up the transect. While the same individuals were potentially detected on the multiple surveys per night or morning and thus leading to non-independence among surveys, it does not bias our data in that simple species presence or absence was used for our species richness and community composition analyses. We ensured that all individuals were within the fragment boundaries by estimating their location in reference to that of the surveyor at various locations on the transect. An individual survey lasted an average of 54 min (range = 35–110 min, SD = 9 min), and each study fragment was surveyed on three randomly ordered nights within a month (i.e. 12 individual surveys over three separate nights per fragment). Survey start times were randomized within evening and early morning time periods, and surveys were not conducted during inclement weather.
Environmental variables
We quantified metrics of forest structure within each fragment, along with fragment-level characteristics such as fragment area and surrounding forest cover. Forest structure variables were measured in 100 contiguous 5 × 5-m plots running the length of the survey transect in each fragment (Browne & Karubian Reference BROWNE and KARUBIAN2016). Values were recorded at the centre point of each 5 × 5-m plot, then averaged across the entire transect to provide a mean value for each fragment. Canopy height was estimated using a digital rangefinder as the height of the tallest tree over the plot. We estimated the number of large trees (defined here as trees with diameter at breast height >50 cm) within each plot. Canopy openness was estimated using the methods of Brown et al. (Reference BROWN, JENNINGS, WHEELER and NABE-NIELSEN2000).
For fragment attributes, altitude was recorded with a handheld GPS (Garmin) in each 5 × 5-m plot and then averaged across the entire transect. Area was estimated by walking around the edge of each fragment with the handheld GPS. The amount of forest cover surrounding each fragment was measured using methods of Browne & Karubian (Reference BROWNE and KARUBIAN2016). Ground-truthed remote-sensing imagery from the Global Forest Change dataset (Hansen et al. Reference HANSEN, POTAPOV, MOORE, HANCHER, TURUBANOVA, TYUKAVINA, THAU, STEHMAN, GOETZ, LOVELAND, KOMMAREDDY, EGOROV, CHINI, JUSTICE and TOWNSHEND2013) was used to calculate per cent of forest cover in 2013 within a 1-km radius from the centre point of each fragment.
Species richness
We calculated species richness as a simple count of the number of species recorded in each fragment across all surveys. We used multiple linear regressions with an all-subset model-averaging approach to investigate the relationship between species richness and the environmental predictor variables described above using the R package ‘MuMIn’. The all-subset approach is an inherently exploratory analysis to evaluate all potential combinations of predictor variables and rank top models based on AICc scores (Symonds & Moussalli Reference SYMONDS and MOUSSALLI2011). We next only included in further analyses models with a ∆AICc <10 compared with the top-ranked model, which eliminates models that have little or no support (Burnham & Anderson Reference BURNHAM and ANDERSON2002). We then calculated model-averaged coefficients using conditional averaging (also known as natural averaging), where parameters are averaged across models where they are present (Burnham & Anderson Reference BURNHAM and ANDERSON2002). We elected to use conditional averaging instead of full model averaging (also known as zero averaging) because we were interested in detecting biologically meaningful effects of our environmental variables on species richness, even if these effects might be relatively weak (Grueber et al. Reference GRUEBER, NAKAGAWA, LAWS and JAMIESON2011, Symonds & Moussalli Reference SYMONDS and MOUSSALLI2011). Covariates were mean centred and scaled by dividing by 1 SD to allow comparison between regression coefficients (Schielzeth Reference SCHIELZETH2010). The mean and SD of covariates are provided in Table 2. Correlations among predictor variables ranged from 0.09–0.59. Variance inflation factor (VIF) values for the regression of all predictor variables on species richness were <3 for all predictors, below the threshold suggested by Zuur et al. (Reference ZUUR, IENO and ELPHICK2010) where collinearity among predictor variables would be a cause for concern. Regressions were checked to make sure they did not violate any assumptions of the model (e.g. homoscedasticity, normality of residuals). To determine whether time of survey was associated with species richness, we compared observed species richness of AM and PM surveys within each fragment using a paired t-test.
Table 2. Mean, standard deviation (SD), and units of predictor variables used in species richness and community composition for nocturnal birds in 22 forest fragments in north-west Ecuador. Canopy openness was measured as an index ranging from 1–25 following Brown et al. (Reference BROWN, JENNINGS, WHEELER and NABE-NIELSEN2000).

Community composition
We compared nocturnal bird community composition across fragments with a non-metric multidimensional scaling (NMDS) ordination using Bray–Curtis dissimilarity based on presence/absence data. NMDS is a type of ordination used to visualize similarity between ecological communities (Zuur et al. Reference ZUUR, IENO and SMITH2007). We then examined the fit of environmental variables to the ordination to assess how well these parameters explained similarity in nocturnal bird communities between fragments using the ‘envfit’ function in the R package ‘vegan’. We tested the significance of each environmental covariate via permutation (n = 999).
RESULTS
Species richness
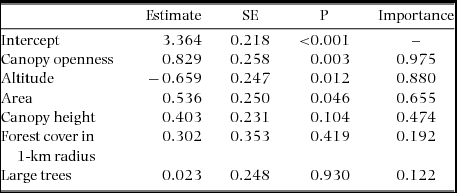
We conducted 264 surveys in 22 forest fragments and recorded a total of 11 species of nocturnal bird (Table 1), with a mean ± SD of 3.4 ± 1.4 (range = 2–7) species per fragment. Among our environmental variables, canopy openness, altitude and fragment area were significantly related to nocturnal bird species richness (Table 3). Canopy openness, an index of light availability and habitat structure, was the strongest predictor of species richness, having the highest magnitude coefficient estimate. More open canopies were linked to higher nocturnal bird species richness. Also, higher species richness was associated with decreased altitude and increased fragment area. Other environmental covariates – canopy height, surrounding forest cover and number of large trees – were weaker predictors of nocturnal bird species richness, having coefficient estimates that did not differ significantly from zero (Table 3).
Table 3. Model-averaged coefficients of linear regressions on nocturnal bird species richness in 22 forest fragments sampled in north-west Ecuador. Canopy openness, altitude and fragment area were the best predictors of nocturnal bird species richness, with largest effect sizes and occurring in the majority of models. Importance is the sum of the Akaike weights over all models in which the covariate appears. Data on the mean and SD of predictor variables are available in Table 2.
Surveys conducted during the early morning period detected more species than did night surveys (0.50 ± 0.17 species per survey vs. 0.30 ± 0.18, respectively; paired t-test: t = 4.67, df = 21, P < 0.001).
Community composition
The similarity of nocturnal bird communities among fragments was not significantly related to any measured habitat variable (P >0.05, Figure 2). The amount of surrounding tree cover, canopy height, fragment area and canopy openness explained relatively little variance (<12% for all) and were not significantly correlated with nocturnal bird community structure (P >0.05, Figure 2).

Figure 2. Visualization of non-metric multidimensional scaling (NMDS) ordination on nocturnal bird species composition with habitat and fragment-level variables in 22 forest fragments of north-west Ecuador. Sampled forest fragments (filled circles) along gradients of environmental variables (blue arrows) (a); individual species (filled circles, some overlapping) and their relation to environmental gradients (b). Fragments that are closer together on the NMDS 1 and NMDS 2 axes share more similar nocturnal bird communities than fragments farther apart, and similarly, species that are closer together are linked to more similar environmental variables than those farther apart. The axes and orientation of the plot are arbitrary. The stress value of 0.117 indicates the NDMS ordination has a reasonably good fit (≤0.20).
DISCUSSION
The over-arching goal of the current study was to identify environmental factors that influence the diversity and species composition of nocturnal birds in a fragmented landscape in north-west Ecuador. An ancillary goal was to provide benchmark information on nocturnal bird populations in these poorly known and highly threatened forests. We distinguished between fine-grained factors related to within-patch habitat quality vs. broader environmental factors such as fragment size, surrounding tree cover and altitude. Our findings indicate that nocturnal bird diversity is shaped by a combination of canopy openness, a fine-grained factor, and two broader environmental factors, fragment size and altitude.
Habitat characteristics and fragment attributes
We found higher nocturnal bird species richness in forest fragments characterized by more open canopies. Although open canopies have the potential to increase detection bias, the vast majority of individuals were detected via vocalization (not sighting), suggesting that this potential source of bias was unlikely to have influenced our findings. Canopy openness is often associated with increased bird species richness, density, diversity and reproduction efforts (Durães et al. Reference DURÃES, CARRASCO, SMITH and KARUBIAN2013, Vázquez-Pérez et al. Reference VÁZQUEZ-PÉREZ, ENRÍQUEZ, RANGEL-SALAZAR and CASTILLO2011, Walter & Maguire Reference WALTER and MAGUIRE2005). More open canopies may also reflect more structurally complex habitats (vs. habitat structure in uniformly closed canopies), which in turn may support a larger number of nocturnal bird species (Esclarski & Cintra Reference ESCLARSKI and CINTRA2014, Lambert & Adler Reference LAMBERT and ADLER2000, Sekercioglu Reference SEKERCIOGLU2010). In the current study, we propose that the higher occurrence in areas with relatively more open canopies may have been related to foraging ecology and prey densities. In forested ecosystems, open canopies are often associated with increased habitat complexity (Hubbell et al. Reference HUBBELL, FOSTER, O'BRIEN, HARMS, CONDIT, WECHSLER, WRIGHT and LOO DE LAO1999, Walter & Maguire Reference WALTER and MAGUIRE2004) that may support increased densities of small mammals (Carvajal & Adler Reference CARVAJAL and ADLER2008, Lambert & Adler Reference LAMBERT and ADLER2000) and insects that serve as prey for nocturnal birds (Halaj et al. Reference HALAJ, ROSS and MOLDENKE2000, Tews et al. Reference TEWS, BROSE, GRIMM, TIELBÖRGER, WICHMANN, SCHWAGER and JELTSCH2004). Treefall gaps also provide open areas that may allow aerial-foraging birds more space to capture prey (Ibáñez et al. Reference IBÁÑEZ, RAMO and BUSTO1992, Ochoa Reference OCHOA2000, Sekercioglu Reference SEKERCIOGLU2010). Future studies focused on foraging ecology would be useful in distinguishing between these putative ecological relationships underlying the richness patterns we present here.
We also detected higher diversity of species in larger fragments. Smaller forest fragments are susceptible to deterioration of habitat conditions due edge effects or invasive species (Ewers & Didham Reference EWERS and DIDHAM2006, Laurance et al. Reference LAURANCE, LOVEJOY, VASCONCELOS, BRUNA, DIDHAM, STOUFFER, GASCON, BIERREGAARD, LAURANCE and SAMPAIO2002). As a result, forest fragmentation is typically associated with reduced bird species richness and diversity in smaller fragments (Durães et al. Reference DURÃES, CARRASCO, SMITH and KARUBIAN2013, Marini Reference MARINI2001, Rolstad Reference ROLSTAD1991). This is particularly true for apex predators like diurnal raptors (Ribon et al. Reference RIBON, SIMON and DE MATTOS2003), and is also likely to apply to nocturnal birds of prey. Our findings of more species detected in relatively larger fragments may be due in part to greater resources, particularly among highly mobile owl species that may require a considerable area to meet resource needs. However, nocturnal birds may also occur in relatively small forest patches while obtaining supplemental resources in nearby vast expanses of old-growth rain forest (Sberze et al. Reference SBERZE, COHN-HAFT and FERRAZ2010, Sekercioglu Reference SEKERCIOGLU2010). The fact that we obtained a significant relationship with fragment area, whereas Sberze et al. (Reference SBERZE, COHN-HAFT and FERRAZ2010) and Sekercioglu (Reference SEKERCIOGLU2010) did not, may be because forest fragments in our study area were often far (>10 km) from large areas of contiguous forest. We detected one additional nocturnal species for every 5.3 ha (95% CI = 0.12–10.5 ha) increase in fragment size.
In addition to canopy openness and fragment size, altitude was also related to species’ distributions in our study area. We found higher numbers of species in lower-altitude fragments; an approximately 100-m decrease in altitude resulted in a predicted increase of one additional nocturnal bird species detected. Our finding, across a gradient of 135 to 592 m asl, corroborates reports of greater owl species richness at lower altitudes in Ecuador (Freile et al. Reference FREILE, CASTRO and VARELA2012). Other assessments found either species-specific relationships (e.g. in Chilean forests; Ibarra et al. Reference IBARRA, MARTIN, ALTAMIRANO, VARGAS and BONACIC2014) or no strong patterns (e.g. in lowland Brazilian forests; Esclarski & Cintra Reference ESCLARSKI and CINTRA2014), but these studies were confounded with habitat quality across altitudinal gradients (Ibarra et al. Reference IBARRA, MARTIN, ALTAMIRANO, VARGAS and BONACIC2014) or limited to a narrower altitudinal range (i.e. 46–105 m; Esclarski & Cintra Reference ESCLARSKI and CINTRA2014). Our findings are in line with studies on diurnal birds (Blake & Loiselle Reference BLAKE and LOISELLE2000) that show species richness commonly increases with decreasing altitude, perhaps because of increased primary productivity and greater prey abundances at lower altitudes (Janes Reference JANES1994).
We did not find any relationship between species richness and amount of surrounding forest cover, counter to previous studies reporting that abundance for many tropical forest-dependent bird species in forest fragments can be influenced by matrix habitat (Cardoso da Silva et al. Reference CARDOSO DA SILVA, UHL and MURRAY1996, Levey et al. Reference LEVEY, BOLKER, TEWKSBURY, SARGENT and HADDAD2005, Prevedello & Vieira Reference PREVEDELLO and VIEIRA2010). Interestingly, concurrent sampling of large-bodied avian frugivores in the same fragments showed that, unlike nocturnal birds, frugivore richness responded strongly and positively to surrounding forest cover (Walter et al. Reference WALTER, BROWNE, FREILE, OLIVO, GONZÁLEZ and KARUBIAN2017). These discrepancies between guilds suggest that additional work evaluating how ecological interactions and usage patterns within the matrix may vary among guilds will be useful for improving our mechanistic understanding of the impacts of fragmentation.
Conclusions
As the first study of its type in the region, this work provides a useful benchmark for nocturnal bird communities in and around the Mache-Chindul Ecological Reserve, a BirdLife International Important Bird Area that supports >250 species of birds and where the majority of previous continuous rain forest has been cleared for agriculture (Browne & Karubian Reference BROWNE and KARUBIAN2016, Carrasco et al. Reference CARRASCO, BERG, LITZ, COOK and KARUBIAN2013, Durães et al. Reference DURÃES, CARRASCO, SMITH and KARUBIAN2013). Two of the species we recorded, the Chocó poorwill (Nyctiphrynus rosenbergi) and Colombian screech owl (Megascops colombianus), are considered ‘near threatened’. Although the Chocó poorwill is reasonably common in lowland forests of north-west Ecuador (Jahn Reference JAHN2011, J.F. pers. obs.), the fact that we recorded the species only once over 5 mo suggests that the species does not cope well with forest fragmentation. Our observations of the Colombian screech owl, which we recorded in three fragments, further supports a range expansion and potential resident population in the Mache Chindul region, where it was only recently detected (Carrasco et al. Reference CARRASCO, COOK and KARUBIAN2008). We did not identify any unique species in Bilsa, the large continuous forest that we surveyed, highlighting the importance of the fragments for conservation of many nocturnal birds.
Given the logistical difficulties associated with sampling nocturnal birds (Lloyd Reference LLOYD2003), we close by providing the following practical suggestion for study design of future and similar research: surveys focused in the early morning may maximize efficiency. In other studies, nocturnal bird surveys are typically conducted from 18h00 to 24h00 (Esclarski & Cintra Reference ESCLARSKI and CINTRA2014, Lloyd Reference LLOYD2003, Ortiz-Pulido & Lara Reference ORTIZ-PULIDO and LARA2014, Sberze et al. Reference SBERZE, COHN-HAFT and FERRAZ2010), and studies that conducted surveys in both evenings and early morning did not compare detections between the two time periods (Ibarra et al. Reference IBARRA, MARTIN, ALTAMIRANO, VARGAS and BONACIC2014, Penteriani et al. Reference PENTERIANI, DELGADO, CAMPIONI and LOURENÇO2010, Vázquez-Pérez et al. Reference VÁZQUEZ-PÉREZ, ENRÍQUEZ, RANGEL-SALAZAR and CASTILLO2011). We recorded 1.2 more species on average during the early morning sampling period (midnight until 6h45) than during the late night period (18h36 to 23h35), and there was only one species that was only detected during nighttime sampling (Chocó poorwill); as this was the only time it was detected in the entire study, it could be just be chance that it was detected during this time period.
ACKNOWLEDGEMENTS
We thank the Ecuadorian Ministry of the Environment and landowners in and around the Mache-Chindul Reserve for access to land and support in the field, and O. Jahn and E. Guevara for initial help in designing field protocols to study nocturnal birds. Fundación para la Conservación de los Andes Tropicales, especially M. González and K. Pozo, and Tulane University provided logistical support. The work was supported by the Conservation Food and Health Foundation; Conservation, Research and Education Opportunities International; Disney Conservation Fund; National Science Foundation (EAGER #1548548, DDIG #1501514, L.B. Graduate Research Fellowship); Ornithological Council; Tulane University; and the United States Fish and Wildlife Service Neotropical Migratory Bird Act (NMBCA #5605). All work was conducted under permits from the Ecuadorian Ministry of the Environment (#010-2014-IC-FLO-FAU-DPE-MA).







