Introduction
A central feature of the postnatal development of mammalian central visual pathways is the existence of critical or sensitive periods during which neural maturation and function are particularly sensitive to visual experience. The profiles of these critical periods at key stages of the visual pathway, as well as their relation to the time course of normal maturation, were documented first in cats and monkeys. Although for some aspects the profiles differed between the two species, particularly, with respect to their duration, there were certain common features. As reviewed by Daw (Reference Daw2006), critical period profiles in both species varied according to the nature of the experimental alteration of the early visual input, the level in the visual pathway, whether they defined the timing of susceptibility to the abnormal visual input (a critical period for damage), or by the capacity for recovery from a prior period of early deprivation following appropriate visual exposure (a critical period for recovery).
The most studied experiential alteration of the early visual input in animals is monocular deprivation (MD), where one eye is deprived of patterned visual input. The large and well-documented anatomical and physiological changes so induced in the visual cortex of cats and monkeys are accompanied immediately by visual deficits in the deprived eye that are so profound as to render the eye effectively blind (Giffin & Mitchell, Reference Giffin and Mitchell1978; Mitchell, Reference Mitchell1988; Harwerth et al., Reference Harwerth, Smith, Boltz, Crawford and Von Noorden1983). Plasticity of the developing visual cortex as revealed by its response to early MD can also be demonstrated by the capacity to recover from visual deprivation following the restoration of binocular visual exposure at different times afterward. The relation between the profiles of critical periods in cats and monkeys in response to the alteration of the visual input to the time course of functional maturation of the visual pathways in normal animals find close parallels with the time course of the development of various visual functions in normal children and the abnormal development reported in children born with a cataract in one eye (Lewis & Maurer, Reference Lewis and Maurer2005).
Critical periods in the visual pathways share in common an arc of vulnerability to particular atypical or biased early visual exposure that starts at birth or shortly thereafter, rises quickly to a peak level of sensitivity, and is followed by a gradual decline. Studies to determine the time course of vulnerability to a particular visual exposure are best exemplified by an experimental design that employs a constant duration of selected visual input imposed on, otherwise, typically reared animals of different ages. In cats, vulnerability to a 10 day period of MD was shown by Olson and Freeman (Reference Olson and Freeman1980) to be manifested first at 2 weeks of age and to attain peak sensitivity at 4–5 weeks followed by a decline to a low but still measurable level at 4 months. Later studies that employed longer (one to three month) periods of MD indicate that vulnerability extends to between 6 and 8 months of age (Cynader et al., Reference Cynader, Timney and Mitchell1980; Jones et al., Reference Jones, Spear and Tong1984; Daw et al., Reference Daw, Fox, Sato and Czepita1992). Although the cat visual cortex possesses a remarkable capacity to recover from a prior period of such deprivation following reversal of the deprivation (reverse occlusion), the ability to do so appears to be confined to a shorter period that ends at 14 weeks of age (Blakemore & Van Sluyters, Reference Blakemore and Van Sluyters1974). The limited number of studies conducted on nonhuman primates (macaque monkeys) suggest that the visual cortex is vulnerable to MD to about 2 years of age (LeVay et al., Reference LeVay, Wiesel and Hubel1980) and that the cortical effects of an early period of MD is reversible by occlusion of the good eye during a shorter period that ends by one year of age (Blakemore et al., Reference Blakemore, Garey and Vital-Durand1978).
Simple restoration of the normal visual input to the deprived eye of kittens following MD to allow visual input to both eyes (binocular recovery) results in incomplete anatomical and physiological recovery in the visual pathways (Baker et al., Reference Baker, Grigg and Von Noorden1974; Hubel et al., Reference Hubel, Wiesel and Levay1977; Mitchell et al., Reference Mitchell, Cynader and Movshon1977; Blasdel & Pettigrew, Reference Blasdel and Pettigrew1978; Olson & Freeman, Reference Olson and Fremman1978; LeVay et al., Reference LeVay, Wiesel and Hubel1980; Blakemore et al., Reference Blakemore, Vital-Durand and Garey1981) as well as limited recovery of vision in the deprived eye (Mitchell et al., Reference Mitchell, Cynader and Movshon1977; Giffin & Mitchell, Reference Giffin and Mitchell1978; Mitchell, Reference Mitchell1988). Even less recovery has been reported in monkeys when binocular visual input is restored after MD (Blakemore et al., Reference Blakemore, Vital-Durand and Garey1981; Garey & Vital-Durand, Reference Garey and Vital-Durand1981; Swindale et al., Reference Swindale, Vital-Durand and Blakemore1981). A remarkable recent advancement has been the discovery in kittens (Duffy & Mitchell, Reference Duffy and Mitchell2013; Mitchell et al., Reference Mitchell, Macneil, Crowder, Holman and Duffy2016) that a relatively short (10 day) period of complete darkness introduced 8 weeks after an early period of MD could boost the visual acuity of the deprived eye to normal levels in just a week. The fact that these kittens were 14 weeks old when darkness was introduced raises the possibility that a 10 day period of darkness may induce recovery at any age. Although this suggestion flies in the face of the strong evidence for critical periods in the kitten’s visual cortex, it is important to note that a prior study conducted on adult Long Evans rats (He et al., Reference He, Ray, Dennis and Quinlan2007) reported fast visual recovery following exposure to darkness. To establish whether the benefits of a short duration of darkness in kittens were restricted to a critical period, we studied the ability of the same 10 day period of darkness to promote behavioral and anatomical recovery in adult cats at one year of age. We found neither behavioral nor anatomical evidence that 10 days of dark exposure reintroduced plasticity in the central visual pathways at one year of age, a result that implies that brief periods of darkness promote substantial improvement of the vision of the deprived eye only when imposed during an earlier critical period.
Materials and methods
Animals
This study was conducted on 18 cats that were born and raised in a closed breeding colony at Dalhousie University. The rearing, housing, and experimental procedures followed protocols that were approved by the Dalhousie University Committee on Laboratory Animals and that conformed to the principles and guidelines regarding the care and use of animals adopted by the American Physiological Society, the Society for Neuroscience, and the Canadian Council on Animal Care.
Animals were reared to address whether a 10 day period of darkness imposed in adulthood could promote recovery from an early period of MD. The extent of any darkness-induced recovery was assessed by behavioral measurements of visual acuity and by anatomical studies of the dorsal Lateral Geniculate Nucleus (dLGN). Experiments were conducted in parallel on separate groups of animals. The 18 animals employed for this investigation included 10 that were monocularly deprived specifically for the anatomical studies as well as an additional 3 normally reared animals that served as controls at either 22 weeks (C371 and C372) or at 28 weeks of age (C246). The five cats that participated in the behavioral studies included two that had been reared specifically for a completed study (C191; Mitchell et al., Reference Mitchell, Macneil, Crowder, Holman and Duffy2016) or for a study in progress (C218). A detailed list of the timing of the various experiential manipulations for all the animals that participated in the study is provided in Table 1. The five animals reared to examine the ability of darkness to promote behavioral recovery in adult cats received an early period of MD from P7 (N = 2) or P30 (N = 3) that lasted till either P37 (N = 4) or P43 (N = 1) days and was sufficiently long to produce deep amblyopia in the deprived eye. The 10 days of dark exposure was imposed at between 13 and 14 months of age in 4 animals and at 8.5 months for the fifth. The dark exposure thus occurred well beyond the 6–8 month upper age estimate for the critical period of vulnerability of the visual cortex to MD (Jones et al., Reference Jones, Spear and Tong1984; Daw et al., Reference Daw, Fox, Sato and Czepita1992).
Table 1. The timing (postnatal days of age) of the periods of monocular deprivation (MD), binocular visual exposure (BV) and darkness for each animal, separated according to the experimental approach (behavior or anatomy)
Because of the extensive and fast anatomical recovery from an early period of MD observed in the dLGN subsequent to restoration of the binocular visual input (O’Leary et al., Reference O’Leary, Kutcher, Mitchell and Duffy2012), the initial period of MD for the animals employed in the anatomical studies of the possible benefits of darkness was longer than that employed for the behavioral studies. Thus, although initiated at a similar age (P30), the period of MD for the anatomical studies of darkness-induced recovery extended far longer, from 3 to 12 months of age. An anatomical parallel study that complemented the behavioral investigation of the ability of darkness to promote vulnerability to MD in adulthood was published recently (Duffy et al., Reference Duffy, Lingley, Holman and Mitchell2016).
Darkroom facility
The darkroom facility was constructed inside a large windowless room and consisted of two adjacent rooms for the housing of animals separated by two light-tight doors so that animals could be moved from one room to the other to allow their holding room and cages to be cleaned and their food and water bowls to be replenished.
A complete description of the darkroom facility including the dimensions of the rooms and the cages is provided in a recent article (Mitchell, Reference Mitchell2013). The design of the facility incorporates features to ease both the daily feeding of the animals and regular cleaning of the holding room and their cage(s), while maintaining the animals in complete darkness. To ensure that all light is excluded during entry of the personnel required for these tasks, the entrance into the darkroom in which the animals are housed is made through one or two dark adjacent anterooms that are separated from the illuminated exterior of the facility by two to three light-tight doors.
Behavioral methods
Measurements of the visual acuity for square-wave gratings were made by making use of a jumping stand and procedures that have been refined over the last four decades. Detailed descriptions of the jumping stand and the current training and testing procedures are provided in several recent articles (Mitchell, Reference Mitchell2013; Mitchell et al., Reference Mitchell, Macneil, Crowder, Holman and Duffy2016). Here we describe the main features of the method together with the documentation of the visual characteristics of the stimuli. Animals were trained as kittens when 4–5 weeks old on a jumping stand to discriminate between vertical and horizontal square-wave gratings having the same large period (32 mm). The height from which the kittens jumped was raised over time to commensurate with their motor skills beginning from just a few cm to an eventual height of 72 cm at about 6–8 weeks of age. Once trained on the basic discrimination, measurement of visual acuity was made by use of a series of gratings of gradually decreasing periods between blocks of trials. The period of the gratings was decreased in very small but equal logarithmic steps with as many as 12 steps per octave change of period. At a constant jumping height, the use of such a series resulted in gradual and equal logarithmic changes of the grating spatial frequency. The use of such steps in grating size finds a parallel with the design of modern Logmar acuity charts, where the letter size change between rows is also equal and logarithmic but with only three steps per octave. At the start of a test session, each block of trials consisted of as few as one trial, but within an octave (a factor of two) of the threshold, a minimum five of trials was provided at each spatial frequency. After an error, the animal was required to correct its response and then to achieve either 5 consecutively correct responses or a minimum of 7 correct out of a maximum of 10 trials at any spatial frequency, before moving on to presentations of a higher spatial frequency. All the training and testing was conducted by two people who sat on either side of the jumping stand. Neither person was blind to the rearing history of the animals.
As a possible consequence of the very gradual and equal changes in the degree of difficulty of the task, the acuity could be titrated precisely and with a high degree of repeatability. The performance was usually flawless until the final few incremental steps of spatial frequency when the animal’s performance fell to chance accompanied by overt signs of difficulty such as crying, frequent looking at adjacent objects, and increased response latency before its jump. Measurements were made of the acuity of the deprived eye at very regular intervals (approximately weekly) in their first year in order to ensure that the animals continued to perform well on the discrimination. However, testing was conducted every one or two days in the two weeks prior to and following dark exposure. Because of the long interval between the early period of MD and the exposure to darkness, the animals received many more trials prior to the latter intervention than in our prior studies. The one exception was C173 for which measurements of acuity could not be scheduled on a regular basis prior to the period of darkness, and as a possible consequence, acuity measures for either eye made in the immediate aftermath of the dark exposure at P389 days were poor. As a consequence, it was necessary to retrain the animal so that reliable measurements of acuity of either eye could not be made for 6 weeks.
Monocular deprivation
MD was performed by procedures developed to both secure the eyelid closure and allow fast recovery of a normal palpebral aperture subsequent to reopening so as to facilitate longitudinal behavioral measurement of the visual acuity of this eye (Murphy & Mitchell, Reference Murphy and Mitchell1987). The eyelid suture of the left eye was performed under general gaseous anesthesia (3–4% isoflurane in oxygen) and was conducted in two stages. First, animals received a drop of a sterile local ophthalmic anesthetic, Alcaine (proparacaine hydrochloride), onto the cornea of the left eye and after which the palpebral conjunctivae from the upper and lower eyelids were dissected free from the eyelids and then sutured together with the 6-0 vicryl suture material. A broad-spectrum topical antibiotic (Chloromycetin 1%) was applied to the sutured conjunctivae prior to the second surgical stage of the procedure in which the exposed tissue on the underside of the eyelids was opposed and sutured together with 6-0 or 5-0 silk. Following the surgery, the animals were administered subcutaneous injections of Ketoprofen for postprocedure analgesia and a broad-spectrum topical antibiotic (1% Chloromycetin) to mitigate any infection. At the end of the period of MD, the eyelids of the deprived eye were parted gently under general gaseous anesthesia.
To minimize any unwanted visual exposure, the cats that received a long period of MD prior to darkness (C170, C171, C203, C206) were placed in the darkroom with the eyelids of one eye still closed. At the end of the period of darkness, they were transported by a light-tight carrier from the darkroom to the animal surgical suite, where they were placed immediately into a darkened anesthetic induction box prior to the eyelids being opened. Any light exposure prior to surgery would have been of very low intensity and brief, lasting in total less than 2 min.
Anatomical methods and quantification
Cats were deeply anesthetized with isofluorane (3–4% in oxygen) prior to being the receipient of a lethal injection (IP) of sodium pentobarbital. Subsequently, the animals were perfused transcardially to exsanguinate the brain tissue with 250–350 ml phosphate buffered saline (PBS, 0.1 M, 4°C, pH 7.4) followed by 250–350 ml of 4% paraformaldehyde. The brain was removed from the cranium and dissected to reveal the thalamus following which the occipital, frontal, and temporal lobes were removed from each hemisphere. The resulting block of the thalamus contained both left and right dLGNs and was placed in 30% sucrose in 0.1 M PBS at 4°C to establish cryoprotection for microtomy. Each tissue block was embedded in Tissue Freeze (Triangle Biomedical; Durham, NC) and mounted on a freezing microtome (Leica SM2000R; Nussloch, Germany), where 50 µm thick coronal sections were cut through the entire dLGN. Alternate sections were prepared for Nissl staining and for the immunohistochemical reaction for Neurofilament-H (NF-H). Sections prepared for the Nissl stain were wet mounted onto glass slides, allowed to dry overnight, dehydrated using a graded dilution series of ethanol, and then placed in cresyl violet for 5 min. Sections were then differentiated using the ethanol dilution series to optimize the staining of perikaryon, cleared in Histo-clear (DiaMed Lab Supplies Inc.; Mississauga, ON, Canada) and coverslipped with permount (Fisher Scientific; Ottawa, Canada). Tissue sections adjacent to those used for Nissl staining were left free-floating in PBS and probed for the presence of Neurofilament-H (NF-H) using the monoclonal SMI-32 antibody (Covance, 1:1000) that targets a nonphosphorylated epitope of the heavy chain subunit of the neurofilament (NF) protein. Antibody specificity was verified using immunoblots of a cat’ visual cortex that gave rise to a single band of immunoreactivity at approximately130 kDa, congruent with the previously published data on NF-H (Julien & Mushynski, Reference Julien and Mushynski1982; Kaufmann et al., Reference Kaufman, Geisler and Weber1984; Georges & Mushynski, Reference Georges and Mushynski1987). Sections from each animal in this study were reacted at the same time, with the same reagents, and for the same duration so as to minimize variability between animals. Sections were incubated overnight at 4°C in SMI-32, rinsed with PBS, and incubated in a secondary antibody (Jackson Imunolabs: 115-065-146, 1:1000) conjugated with an avidin-biotin complex using a Vectastain ABC kit (Vector Laboratories; Burlingame, CA). Antibodies were then made visible using the 3′3-diaminobenzadine tetrahydrochloride chromagen. Reacted sections were mounted onto glass sides, dried overnight, dehydrated with ethanol, cleared in Histo-clear, and cover slipped using permount.
Measurements of the cross-sectional area of cell bodies were performed separately in layers A and A1 of the right and left dLGN by use of the nucleator stereology probe (newCAST; VisioPharm, Hörsholm, Denmark). Only those cells exhibiting a darkly stained cytoplasm and a weakly stained nucleus were measured, ensuring that the measurements were limited to the neurons that had been cut through the presumed soma midline and avoided the inclusion of measurements from glial cells. Neurons were made visible at 600× magnification using a B×51 compound microscope fitted with a high-resolution DP-70 digital camera (Olympus, Markham, Canada). Measurements were taken from two to three sections per animal yielding a minimum of 1458 cells measured per animal.
The density of NF-H label was quantified by stereologically counting positively labeled neurons within the defined regions of interest. Positively labeled neurons exhibited a uniformly dark cytoplasm around a light nucleus. Counts were performed under the same conditions as those employed for the cell soma measurements by use of an optical dissector probe from a computerized stereology program (newCAST; VisioPharm, Denmark), which generated a random 50% surface area sample of each A and A1 layer of the right and left dLGN.
Within—animal comparisons were made between measurements taken from the A and A1 layers of the dLGN both for the cell size and density by use of a deprivation metric (1).
The deprivation metric has a value near zero when no differences exist between values measured in the deprived and nondeprived layers, as observed in normal animals (Kutcher & Duffy, Reference Kutcher and Duffy2007; Duffy & Slusar, Reference Duffy and Slusar2009). A positive value indicates that measures taken from the deprived layers are less than those in the nondeprived layers, while a negative value indicates that deprived measures are greater than nondeprived. More detailed descriptions of the anatomical methods and analysis are provided in several recent articles (O’Leary et al., Reference O’Leary, Kutcher, Mitchell and Duffy2012; Duffy et al., Reference Duffy, Holman and Mitchell2014b, Reference Duffy, Lingley, Holman and Mitchell2016).
Results
Behavior
Failure of darkness imposed on adult cats to promote recovery from amblyopia
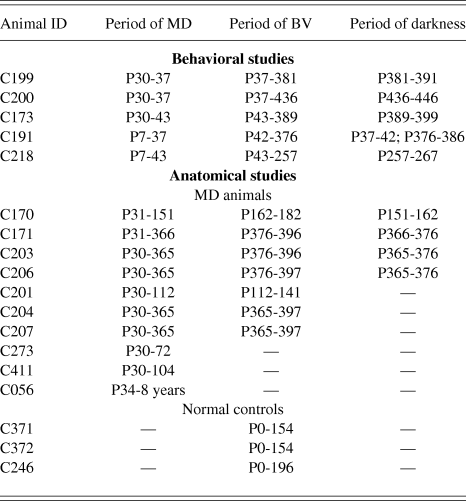
Fig. 1 displays the results of longitudinal measurements of visual acuity made on two amblyopic animals immediately prior to and following 10 days of darkness imposed at either P380 (C199) or P436 (C200) days of age. There was no detectable improvement of visual acuity in the deeply amblyopic eye of either animal in the days following the period of darkness. Measurements of acuity of the deprived eye were continued for 12 (C200) to 20 (C199) days following the dark exposure, a period that extends much longer than the 5–10 days over which acuity recovers following the dark exposure imposed on animals at 3–4 months of age (Mitchell et al., Reference Mitchell, Macneil, Crowder, Holman and Duffy2016). The results from the third animal (C173) that had to be retrained after the period of darkness that was imposed at 389 days of age were in agreement. Reliable measurements of the acuities of the two eyes of this animal made six weeks after the period of darkness (respectively, 7.4 and 2.2 cycles/deg for the non-deprived and deprived eye) were no different from the values obtained beforehand.
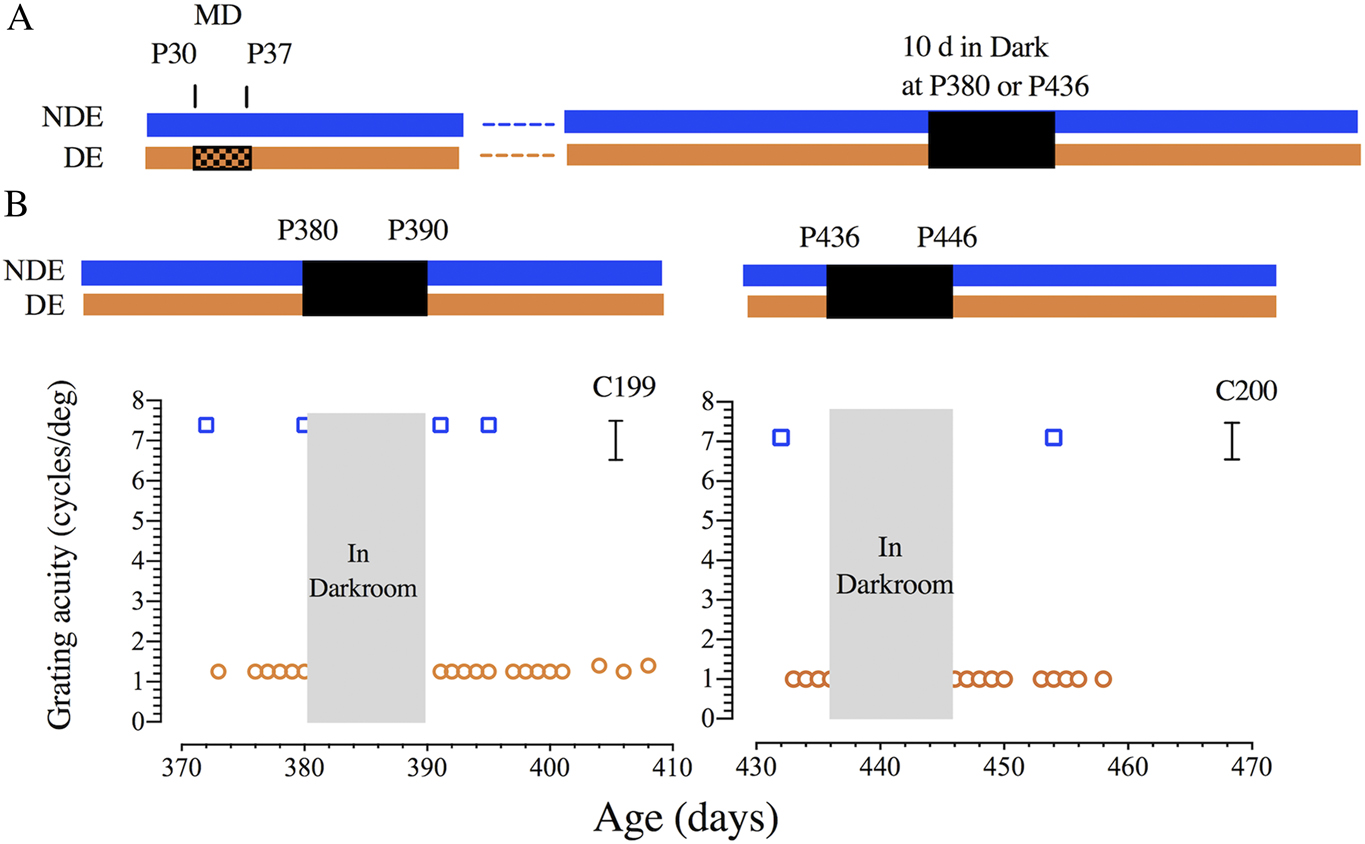
Fig. 1. Failure of 10 days of darkness to promote recovery of vision of the amblyopic eye of two adult cats (C199 & C200). (A) A schematic representation of the visual rearing histories. Both animals received a one week period of MD (shown by hatching) from P30 to P37 followed by one year of binocular visual exposure. At either P380 (C199) or P436 (C200) days of age each animal was placed in complete darkness for 10 days. (B) Graphs showing the results of longitudinal measurements of the binocular grating acuity (blue square symbols) and of the acuity of the amblyopic deprived eye (orange circles) as a function of the animals’ age. Brackets to the right indicate the range of monocular acuities of normal animals from about 4 months of age. Abbreviations: NDE—nondeprived eye; DE—deprived eye.
The results from these 3 animals suggest that 10 days of darkness imposed at one year of age does not promote any recovery of vision in the amblyopic eye. This conclusion receives additional support from the results from a fourth animal (C191) that had participated in an earlier study (Mitchell et al., Reference Mitchell, Macneil, Crowder, Holman and Duffy2016; Fig. 3D) for which 5 days of darkness proved ineffective. On the basis of a prior demonstration (Duffy et al., Reference Duffy, Bukhamseen, Smithen and Mitchell2014a) that 10 days of darkness imposed at 3 months could still promote full recovery even following a prior intervention that proved unsuccessful (binocular eyelid suture), we placed this animal in darkness for 10 days when it was a year of age (P375); Fig. 2 displays the results of measurements of acuity surrounding both periods of darkness. Although the initial 5-day period of darkness imposed immediately following a period of MD from P7 to P37 reduced the vision of both eyes temporarily, only the acuity of the nondeprived eye recovered to normal levels, while the deprived eye achieved only very poor acuity that was not improved by the second period of darkness that occurred at 376 days of age.
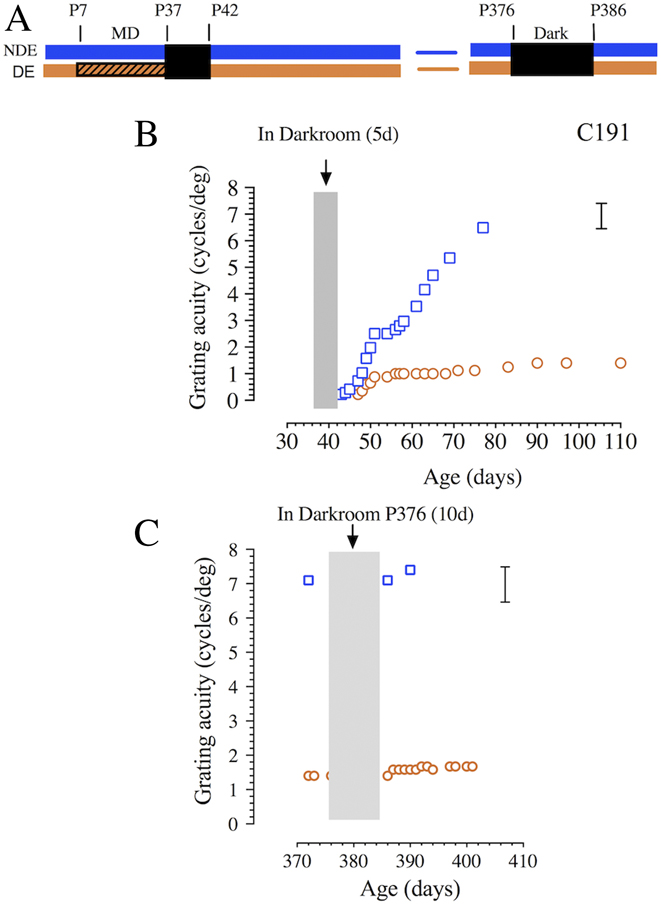
Fig. 2. Failure of a 10 day period of darkness to promote recovery of vision of the amblyopic eye of an adult cat (C191) at P376 days. (A) A schematic representation of the visual rearing history. Following a period of MD from P7 to P37, the animal experienced 5 days of complete darkness followed by one year of binocular visual exposure. At P376 days of age the cat was placed in darkness for a second time for 10 days followed once again by binocular visual exposure. (B) A graph that displays the results of longitudinal measurements of the binocular grating acuity (blue square symbols) and of the acuity of the amblyopic deprived eye (orange circles) as a function of the animals’ age. Data redrawn from Mitchell et al. (Reference Mitchell, Macneil, Crowder, Holman and Duffy2016; Fig. 3D). (C) Longitudinal measurements of the binocular and deprived eye acuity immediately prior to and following a day period of total darkness that began on P376 days. Brackets to the right indicate the range of monocular acuities of normal animals from about 4 months of age. Abbreviations as for Fig. 1.
Finally, the data obtained from a fifth animal (C218, see Fig. 3) that was placed in darkness for 10 days at 8.5 months (at P257) suggests that darkness-induced recovery does not occur even at this age. Although the initial period of MD was imposed at P7 and extended to P43, 10 days of darkness initiated at 3 months of age (P92) on a littermate (C219; Mitchell et al., Reference Mitchell, Macneil, Crowder, Holman and Duffy2016; Fig. 2B) was shown to promote fast and complete recovery of visual acuity in the deprived eye. Thus the lack of visual recovery observed with C218 could not be attributed to the long prior period of MD but rather to the age at which darkness was imposed suggesting that the effectiveness of darkness may be restricted to an early period that terminates at some point between 3 and 8.5 months of age.
Fig. 3. Failure of a 10 day period of darkness to promote recovery of vision of the amblyopic eye of an 8 month-old cat (C218). (A) A schematic representation of the visual rearing history. Following a period of MD from P7 to P43 (hatching), the animal experienced 10 days of complete darkness at P257 days of age followed by binocular visual exposure. (B) Graphs that display the results of longitudinal measurements of the binocular grating acuity (blue square symbols) and of the acuity of the amblyopic deprived eye (orange circles) as a function of the animals’ age. The arrow indicates the first day on which the animal exhibited form vision with its deprived eye. Brackets to the right indicate the range of monocular acuities of normal animals from about 4 months of age.
Anatomy
Failure of darkness imposed at one year to enhance anatomical recovery from MD
The extent of anatomical recovery from an extended early period of MD was examined in the dLGN under two recovery conditions. In one recovery condition, experienced by 3 animals (C201, C204, C207), the long period of MD was followed by 29–31 days of binocular visual input. In the second condition, 4 animals (C170, C171, C203, C206) experienced the same duration of initial MD with the addition of a subsequent recovery period broken into two intervals in which a 10–11 day period of darkness preceded 20–21 days of binocular vision. The morphological consequences of these two rearing conditions as well as those observed following an equivalent period of MD alone were quantified in terms of laminar differences in both the soma cross-sectional area (as revealed in Nissl stained sections) and in the density of the NF-positive neurons (as revealed by immunoreactivity to NF-H).
The results for the two main rearing conditions and their controls in terms of laminar differences in soma cross-sectional areas in the dLGN are displayed in Fig. 4. Periods of MD lasting about one year produced a large reduction of neuron soma size within the deprived eye recipient layers (Fig. 4A) that was exemplified by comparison with the measurements from normal animals (Fig. 4C). Quantification of the laminar differences of the soma area revealed values of between 20 and 35% that mirror both the size and variability reported in animals deprived for similar long periods in the past. The large laminar differences observed after long periods of MD remained unchanged in three animals (C201, C204, C207) that received a month of binocular visual exposure subsequent to the long period of MD (Fig. 4C). Importantly, this was also true for 4 animals that received 10–11 days of darkness prior to the binocular visual experience as evident in the photomicrograph of Fig. 4B as well as in the quantification shown in Fig. 4C. Thus, we found no evidence of any recovery of laminar cell area sizes in any of the conditions examined, including that in which the binocular visual exposure was preceded by 10 days of darkness.

Fig. 4. Dark exposure for 10 days in adulthood does not promote recovery from the MD-induced reduction of neuron somata within deprived dLGN layers. (A) Effects of 1 year MD. Nissl staining within the right and left dLGN following one year of MD revealed a large reduction in the size of neuron somata within deprived-eye layers (asterisks). The boundary between eye-specific layers is indicated with an arrowhead. (B) Effects of 1 year MD + Dark exposure. When a year-long MD was followed by 10 days of dark exposure and subsequent binocular vision, the effect of MD on the dLGN neuron soma size persisted and appeared unchanged, with the originally deprived neurons still substantially smaller than their nondeprived counterparts. (C) Calculation of the deprivation effect between the size of deprived and non-deprived neuron soma size revealed that the 30% reduction in the size of deprived neurons was not changed when the MD was followed either by a period of binocular vision or a period of dark exposure with subsequent binocular vision, both of which also exhibited a deprivation effect that was about 30%. These findings indicate the dark exposure applied in adulthood does not promote recovery from the effect of MD on neuron soma size. Scale bar = 100 µm.
A similar result was apparent from an examination of NF protein, which provides a sensitive marker of the effects of MD and recovery in the dLGN (O’Leary et al., Reference O’Leary, Kutcher, Mitchell and Duffy2012). As is evident from Fig. 5A, the effects of long-term MD on the dLGN was profound with a substantial reduction in the number of NF-H immunoreactive cells within deprived laminae. Quantification revealed an approximate 50% laminar difference in terms of the Deprivation Metric, and there was no evidence of any recovery in the laminar differences with respect to the number of NF-H positive neurons subsequent to either of the two recovery conditions examined (Fig. 5B and 5C). In particular, there was no evidence that 10 days of darkness was able to promote any recovery when applied at about one year of age.
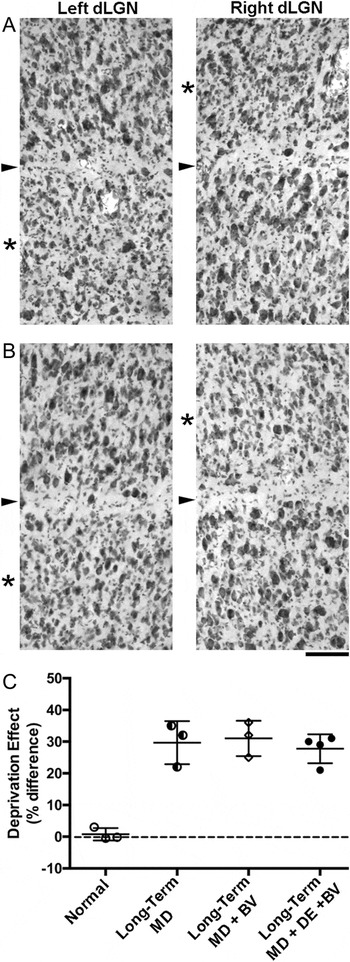
Fig. 5. The substantial loss of neurofilament labeling produced by a year of MD was not altered by 10 days of dark exposure applied in adulthood. (A) Effects of 1 year MD on NF-H label. A large reduction of neurofilament labeling was evident within deprived-eye dLGN layers (asterisks) following a year of MD. The boundary between eye-specific layers is indicated with an arrowhead. (B) Effects of 1 year MD + Darkness on NF-H label. There was no change in neurofilament labeling when 1 year of MD was followed by dark exposure and subsequent binocular vision. The dLGN of animals following darkness and binocular recovery appeared indistinguishable from MD-only controls, both of which showed a large reduction of neurofilament labeling within deprived layers. (C) Stereological measurement of neurofilament-positive cell density enabled calculation of the deprivation effect across conditions and showed that the 50% reduction of neurofilament-positive cell density within deprived dLGN layers was not altered when MD was followed by either binocular vision or dark exposure with subsequent binocular vision. The magnitude of the deprivation effect in both of these recovery conditions was about 50% and therefore equivalent to the MD-only condition. These results indicate that dark exposure in adulthood was not effective at altering the effect of MD. Scale bar = 100 µm.
Discussion
In previous studies (Duffy & Mitchell, Reference Duffy and Mitchell2013), we showed that amblyopia induced by an early period of MD, assessed by measurements of grating acuity, could be eliminated following a 10 day period spent in total darkness. In these prior studies, the period of darkness commenced when kittens were 3 months old, a time well beyond the age (4–5 weeks) of peak susceptibility of ocular dominance of neurons in V1 to the effects of MD (Olson & Freeman, Reference Olson and Freeman1980). The quick and substantial nature of the recovery of visual acuity of the amblyopic eye induced by darkness at 3 months raised the possibility that 10 days of darkness might induce beneficial effects at ages beyond the accepted end of the critical period of susceptibility of the visual cortex to MD at 6–8 months, and possibly even extend into adulthood. The potential benefits of darkness imposed on adult amblyopic cats were raised by observations of substantial darkness-induced recovery of both the physiological and behavioral consequences of MD in adult Long Evans rats (He et al., Reference He, Hodos and Quinlan2006, Reference He, Ray, Dennis and Quinlan2007). Darkness-induced benefits have also been observed in monocularly deprived adult mice. Stodieck et al. (Reference Stodieck, Greifzu, Goetze, Schmidt and Löwel2014) reported a restoration of ocular dominance plasticity in the visual cortex of 4–18 month-old adult mice in response to a 7 day period of MD that was immediately preceded by a 10–14 day period of darkness. A far more modest benefit of darkness was observed in an investigation of the benefits of a week of darkness on the recovery of ocular dominance in the visual cortex of juvenile mice from a month of MD (Erchova et al., Reference Erchova, Vasalauskaite, Longo and Sengpiel2017). To test for possible beneficial effects of darkness in adult cats, we examined the consequences of a 10 day period of darkness imposed on amblyopic animals at one year of age. To aid the comparison with the earlier results (Duffy & Mitchell, Reference Duffy and Mitchell2013; Mitchell et al., Reference Mitchell, Macneil, Crowder, Holman and Duffy2016), the prior period of MD for animals in the behavioral study was initiated at either P7 or P30 days and for the same duration (7 days). The results of both the behavioral and anatomical studies revealed no evidence that a 10 day period of total darkness experienced in adulthood could remediate either the visual or the anatomical consequences to the thalamus of a period of MD imposed in early life.
The failure of 10 days of darkness to elicit any recovery of vision when imposed on adult cats indicates that the benefits of a fixed period of darkness declines with age. However, this finding neither implies that darkness becomes completely ineffective beyond a certain age nor that darkness cannot induce plasticity in the adult cat visual cortex. For example, it is possible that in older cats, darkness has to be combined with another manipulation such as occlusion of the fellow eye or periods of active visual stimulation of the deprived eye in order to produce a beneficial outcome. Another possibility is that darkness might remain effective at any age but that the length of darkness necessary to produce a beneficial change increases with age. The latter interpretation receives support from anatomical studies of the dLGN that indicates that the length of darkness required to reduce laminar differences in cell soma size following early MD increases proportionally to the length of MD, and consequently the age at which darkness is introduced (Kutcher & Duffy, Reference Kutcher and Duffy2007). To gain insight into the apparent decline in the benefits of darkness we have initiated a study to document the rate of decline with age of the efficacy of a fixed 10 day period of darkness imposed on animals that received the same 7 day period of early MD starting at P30 days. The data from C218 (Fig. 3) suggests that 10 days of darkness promotes recovery of the vision of the deprived eye only if imposed on kittens before 8.5 months of age.
The apparent difference in the benefits of 10 days of darkness imposed on rodents and cats in adulthood may not necessarily imply that darkness-induced recovery occurs only during a critical period in cats but can occur at any age in rats or mice. For example, the discrepant results between adult rats and cats may result from the use of the same period of darkness on both species as 10 days may represent a vastly different multiple of the minimum dark period required to produce measurable benefits in rodents versus cats. Studies are in progress to help resolve this issue, including the formal study mentioned earlier of the efficacy of 10 days of darkness as a function of age.
In all our studies, the animals had both eyes open following the period spent in darkness. For kittens placed in darkness prior to 4 months of age, the recovery of the visual acuity of the deprived eye was both fast and complete and unaccompanied by fellow eye grating acuity deficits (Duffy & Mitchell, Reference Duffy and Mitchell2013). Furthermore, without any additional manipulations or training, about one-third of the kittens acquired stereoscopic vision (Mitchell et al., Reference Mitchell, Macneil, Crowder, Holman and Duffy2016). These exciting results hold a promise of translation of a period of darkness to the treatment of amblyopia in children. On the other hand, the same darkness protocol failed to alleviate the visual loss of the deprived eye when administered in adult cats. As mentioned earlier, it could be argued that for the visual acuity of the deprived eye to improve in adult animals, it might be necessary to either increase the length of darkness and/or for the fellow eye to be occluded immediately after the animal was removed from the darkroom. However, on the basis of observations of the consequences of the latter intervention in young animals (Movshon, Reference Movshon1976 a,Reference Movshon b ), occlusion of the fellow eye is likely to result in visual deficits in this eye as well as preventing the acquisition of stereoscopic vision. From the perspective of translation to the treatment of human amblyopia, a short period of darkness applied in childhood holds far more appeal than the potentially longer period necessary for adults, particularly when combined with the potential for reduction in the visual acuity of the fellow eye.
Acknowledgments
Supported by individual Natural Science and Engineering Research Council grants to DEM and KRD as well as by a joint grant to both from the Canadian Institute of Health Research.







