Surgical site infections (SSIs) are among the most common and costly reported healthcare-associated infections.Reference Zimlichman, Henderson and Tamir 1 , Reference de Lissovoy, Fraeman, Hutchins, Murphy, Song and Vaughn 2 However, SSI surveillance systems were designed for, and are primarily focused on, inpatient invasive surgical procedures.Reference Hall, Schwartzman, Zhang and Liu 3 These systems, often heavily reliant on microbiology results, may fail to capture the full range of infectious complications following minor surgical procedures occurring in an ambulatory setting.Reference Schaefer, Jhung and Dahl 4
The Veterans Affairs’ (VA) Surgical Quality Improvement Program (VASQIP) is used by the VA nationally to assess surgical outcomes and improve the quality of surgical care. A similar surveillance system developed in the private sector, the National Surgical Quality Improvement Process (NSQIP), is now used by > 700 hospitals.Reference Irani 5 However, these programs are limited in scope; procedures deemed to be minor and at low risk of adverse events, including many urologic and skin and soft-tissue procedures, are systematically excluded from review.Reference Khuri, Daley and Henderson 6 – Reference Amici, Rogues and Lasheras 8 Thus, the true SSI burden attributable to these perceived low-risk procedures is unknown. In this study, we evaluated the relative burden of SSI in VASQIP-eligible cases versus those cases deemed too minor for systematic review (VASQIP-ineligible) in the ambulatory setting.
Methods
In this retrospective study, we examined outpatient surgeries performed between October 1, 2011, and September 30, 2015, across 11 VA inpatient facilities and 20 VA freestanding ambulatory surgery centers; data for these surgeries were derived from the VA Corporate Data Warehouse. Outpatient surgeries included in the analysis were identified based on current procedure terminology (CPT) codes using the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Program’s 2014 Surgery Flag software. A VASQIP-eligible procedure was defined as an invasive procedure, identified by CPT code, associated with a measurable morbidity or mortality outcome. Eligible procedures were determined by the VA National Surgery Office. 9
A surveillance model was developed based on postoperative healthcare utilization flags (eg, admission, emergency department visit, and/or multiple visits to any surgical clinic) adjusted for patient comorbidities, demographic data, and procedural factors to identify ambulatory surgeries with a high likelihood of an adverse event within 30 days after the procedure.Reference Mull, Itani and Charns 7 The development dataset included manually reviewed cases with and without a healthcare utilization flag. This model was then validated using a subset of charts selected based on the estimated probability an adverse event occurred. For all manual reviews, a trained nurse identified postoperative adverse events, including SSIs based on VASQIP definitions of superficial, deep-incisional, or organ system infections.Reference Khuri, Daley and Henderson 6 Surgeons on the project provided oversight
We compared SSI rates among VASQIP-eligible and -ineligible cases in our chart-reviewed cases using SAS version 9.4 software (SAS Institute, Cary, NC) χ2 tests.
Results
Overall, 1,001,938 surgeries took place in the outpatient setting during the study period, 30% of which were VASQIP eligible. The healthcare utilization flags used in the surveillance model flagged 6.4% of VASQIP eligible cases and 8.7% of VASQIP ineligible cases. To develop and validate the surveillance model, 2,256 outpatient surgeries underwent manual review.Reference Mull, Borzecki, Hickson, Itani and Rosen 10 Overall, 1,018 (45.1%) procedures were eligible for VASQIP review (Table 1). Nearly all skin and soft-tissue procedures (325 of 345, 95%) and nearly half of urologic procedures reviewed were not eligible for VASQIP surveillance.
Table 1. Characteristics of Procedures With and Without Surgical Site Infections in Sample of FY12-15 VA Outpatient Surgeries a
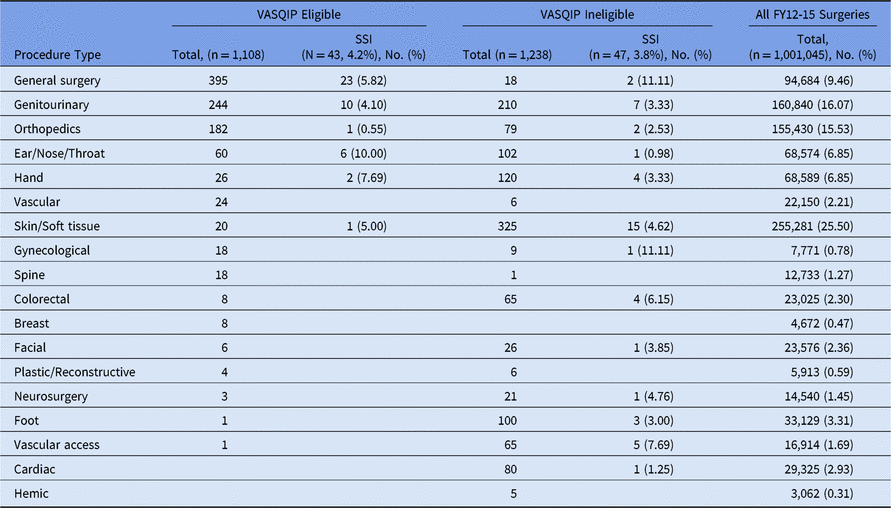
Note: FY, fiscal year; VA, Veteran’s Affairs; VASQIP, Veterans Affairs Surgical Quality Improvement Program; SSI, surgical site infection.
a Data were obtained from development and validation samples for a larger study on an adverse events surveillance model for outpatient surgery.Reference Mull, Itani and Charns7, Reference Mull, Itani and Pizer12 Chart review cases were not sampled to represent surgical specialties.
In total, 90 SSIs (4%) were identified among the manually reviewed cases; 47 SSIs (3.8%) were identified in the VASQIP-ineligible cases compared to 43 SSIs (4.5%) in the VASQIP-eligible cases. The χ2 test result was not significant (P = .61). In line with the rate of adverse event flagging, the distribution of SSIs was 4.6% in skin and soft-tissue cases, 3.7% in urology cases, versus 6.0% in general surgery cases, which would generally be included in standard VASQIP review.
Discussion
This study is the first to evaluate the contribution of minor outpatient procedures to the overall burden of SSI and to evaluate how alternative surveillance strategies based on healthcare utilization rather than more standard microbiology-based surveillance could be used to enhance detection. Using data from the Veterans’ Health Administration, the largest integrated healthcare system in the United States, > 50% of outpatient surgeries reviewed in a prior study of adverse events would not have been considered for SSI review based on current VASQIP policies for clinician-determined operative complexity. Despite the perception of low potential for postoperative adverse events, the contribution of total SSI burden was similar in invasive versus less invasive procedures. This finding suggests that policies around how surveillance is completed in the outpatient environment may need to be re-evaluated.
Although the overall rate of SSI following outpatient surgical procedures is low, the volume and the complexity of these surgeries are rising in parallel. In 2010, there were 28.6 million ambulatory surgery visits to hospital and ambulatory surgical centers (ASCs).Reference Hall, Schwartzman, Zhang and Liu3, Reference Barie11 Despite this increasingly large number of surgical procedures at risk for an adverse event,Reference Hall, Schwartzman, Zhang and Liu3 compliance with current infection prevention standards in outpatient settings has been documented to be as low as 33%.Reference Schaefer, Jhung and Dahl4 The Centers for Medicare and Medicaid Services currently has a voluntary pay-for-reporting program for ASCs to report specific adverse events and has begun to track all postoperative emergency department visits and admissions following surgery in an ASC. However, their current surveillance definition may only account for a small subset of patients undergoing ambulatory procedures within an ASC and not in hospitals. Thus, like VASQIP/NSQIP, this system may not be sufficiently comprehensive to identify and address the full scope of safety concerns. Further study and tracking of postoperative outcomes that includes a broader set of procedures from every specialty performing invasive procedures and all locations of care might allow for targeted infection prevention projects and improvement in overall patient safety for patients receiving care in outpatient settings.
The limitations of this study include use of data from a larger study developing an adverse event surveillance model for outpatient surgeryReference Mull, Itani and Charns 7 because such cases were not sampled to represent surgical specialties. In addition, VA outpatient surgeries include a high prevalence of genitourinary procedures; thus, these results may not be generalizable to non-VA facilities. The strengths of this study include evaluation of a large dataset inclusive of all outpatient surgeries over a 4-year period with manually adjudicated outcomes to provide meaningful information about the scope of SSI in this population.
In conclusion, routine SSI surveillance is limited in the outpatient setting; this gap in care may cause patient safety events to be missed for clinical procedures regarded by clinicians as “safe.” Expanding surveillance and applying utilization triggers to identify adverse events may provide a clearer and more objective picture of the distribution of SSI following outpatient procedures.
Acknowledgments
Statements contained in this article reflect the views of the authors and do not represent the official positions of the US Department of Veterans’ Affairs or other author affiliate organizations.
Financial support
This research was supported by the VA Health Services Research and Development Service (HSR&D) Career Development Award (grant no. CDA 13-270; principal investigator, Mull).
Conflicts of interest
W.B.E. is supported by National Institutes of Health (grant no. NHLBI 1K12HL138049-01). W.B.E. provides expert witness testimony for DLA Piper, LLC. All other authors have no conflicts to report.


