INTRODUCTION
Pollen and seed dispersal influence the spatial distribution, abundance and genetic structure of plant populations (Dick et al. Reference DICK, HARDY, JONES and PETIT2008, Jordano et al. Reference JORDANO, FORGET, LAMBERT, BÖHNING-GAESE, TRAVESET and WRIGHT2011, Wheelwright & Orians Reference WHEELWRIGHT and ORIANS1982). In tropical forests, dioecy and self-incompatibility mechanisms are very common (Ibarra-Manríquez & Oyama Reference IBARRA- MANRÍQUEZ and OYAMA1992), and insects, particularly bees, are the primary pollinators of most tropical tree species (Bawa Reference BAWA1990). Regarding seed dispersal, most tropical woody plant species produce fruits that are primarily dispersed by birds, bats and primates (Arroyo-Rodríguez et al. Reference ARROYO-RODRÍGUEZ, ANDRESEN, BRAVO, STEVENSON, Kowalewski, Garber, Cortés-Ortiz, Urbani and Youlatos2015, Bufalo et al. Reference BUFALO, GALETTI and CULOT2016). Therefore, pollen- and seed-dispersal patterns can be altered in fragmented forests, where defaunation can lead to pollination and seed-dispersal limitation (Cordeiro et al. Reference CORDEIRO, NDANGALASI, MCENTEE and HOWE2009, Cunningham Reference CUNNINGHAM2000, Robertson et al. Reference ROBERTSON, KELLY, LADLEY and SPARROW1999). Unfortunately, pollen- and seed-dispersal distances are difficult to quantify in the field, and are therefore largely unknown for most tropical trees.
Pollen- and seed-dispersal distance are frequently estimated from indirect methods, such as feeding and ranging behaviours of animals, controlled pollination treatments, and seed passage time through the animal gut (Russo et al. Reference RUSSO, PORTNOY and AUGSPURGER2006, Sazan et al. Reference SAZAN, BEZERRA and FREITAS2014). Other studies use different kinds of markers to track pollen and seed movements (Reiter et al. Reference REITER, CURIO, TACUD, URBINA and GERONIMO2006, Webb & Bawa Reference WEBB and BAWA1983). Yet, the development of molecular techniques during the last two decades has facilitated the identification of pollen and seed origins in the field (Dick et al. Reference DICK, ETCHELECU and AUSTERLITZ2003, Godoy & Jordano Reference GODOY and JORDANO2001, Grivet et al. Reference GRIVET, SMOUSE and SORK2005, Hoban et al. Reference HOBAN, SCHLARBAUM, BROSI and ROMERO-SEVERSON2012), enabling accurate quantifications of the pollen- and seed-dispersal distances. However, only one study of primates has used genetic methods to identify the maternal origin of Myrica rubra seeds found in the faeces of the Yakushima macaque (Terakawa et al. Reference TERAKAWA, ISAGI, MATSUI and YUMOTO2009).
Here, we used molecular markers to identify the parental origin of 177 Spondias radlkoferi (Anacardiaceae) seeds dispersed by the spider monkey (Ateles geoffroyi) in two continuous forest sites and two forest fragments in the Lacandona rain forest, Mexico. As an important proportion of dispersed seeds are defecated by this primate in latrines located beneath sleeping-trees (González-Zamora et al. Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, ESCOBAR, RÖS, OYAMA, IBARRA-MANRÍQUEZ, STONER and CHAPMAN2014, Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, ESCOBAR, OYAMA, STONER and AURELI2015; Velázquez-Vázquez et al. Reference VELÁZQUEZ-VÁZQUEZ, REYNA-HURTADO, ARROYO-RODRÍGUEZ, CALMÉ, LÉGER-DALCOURT and NAVARRETE2015), we assessed the parental origin of seeds located in 17 spider monkey latrines to infer potential pollination and seed-dispersal limitation in fragmented forests. Such information is useful to better understand the role of latrines in forest regeneration (Russo & Chapman Reference RUSSO, CHAPMAN, Campbell, Fuentes, MacKinnon, Panger and Bearders2011), as seedling recruitment can be enhanced if seeds come from different parents and have diverse genotypes (Jordano Reference JORDANO, Dennis, Green, Schupp and Wescott2007, Terakawa et al. Reference TERAKAWA, ISAGI, MATSUI and YUMOTO2009). We therefore used nuclear microsatellite markers, which provide a wealth of information about pollen- and seed-mediated gene flow in plants. We identified all candidate parental trees of each seed, to then quantify mean parent-offspring (PO) distances per latrine as a proxy of seed-dispersal distance. When more than one candidate parent emerged for a given seed, we calculated the parent-parent (PP) distance and estimated the mean PP distance per latrine as a proxy of pollen-dispersal distance, and tested if PO and PP distances differed between forest types. We hypothesized that the scarcity of adult trees in fragmented rain forests (Arroyo-Rodríguez & Mandujano Reference ARROYO-RODRÍGUEZ and MANDUJANO2006) may ‘force’ bees to travel longer distances than in continuous forests, thus increasing PP distances (Dick et al. Reference DICK, ETCHELECU and AUSTERLITZ2003, Reference DICK, HARDY, JONES and PETIT2008; Stacy et al. Reference STACY, HAMRICK, NASON, HUBBELL, FOSTER and CONDIT1996). In contrast, because the home range size of spider monkeys is smaller in fragments than in continuous forest areas (Chaves et al. Reference CHAVES, STONER and ARROYO-RODRÍGUEZ2012), we can expect that PO distances are lower in fragmented than in continuous forests.
METHODS
Study area
The Lacandona rain forest constitutes the south-western sector of the Mayan forest in Mexico. The area was originally covered by over 1.4 million ha of rain forest, but deforestation during the last four decades has resulted in the loss of ~70% of the original forest cover. We conducted the study in two adjacent areas separated by the Lacantún river: the Marqués de Comillas region (MCR) and the Montes Azules Biosphere Reserve (MABR) (Figure 1). MCR encompasses c. 176,000 ha of fragmented forest, human settlements and agricultural lands. The study fragments were isolated 25–29 y ago and are located within an anthropogenic matrix of cattle pastures and agricultural lands (González-Zamora et al. Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, OYAMA, SORK, CHAPMAN and STONER2012, Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, ESCOBAR, RÖS, OYAMA, IBARRA-MANRÍQUEZ, STONER and CHAPMAN2014). In contrast, MARB comprises c. 331,000 ha of continuous and undisturbed old-growth forest. Further details on the study landscape are given in González-Zamora et al. (Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, OYAMA, SORK, CHAPMAN and STONER2012, Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, ESCOBAR, RÖS, OYAMA, IBARRA-MANRÍQUEZ, STONER and CHAPMAN2014, Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, ESCOBAR, OYAMA, STONER and AURELI2015).

Figure 1. Location of study areas in the Lacandona rain forest, Mexico: the Marqués de Comillas region (MCR) and the Montes Azules Biosphere Reserve (MABR) (a). The location of the spider monkey latrines (L) and all adult trees of Spondias radlkoferi sampled in two continuous forest sites (CF1 and CF2) and two forest fragments (FF1 and FF2) is also indicated (b). The candidate parental trees of the seeds collected within a given latrine show the same icon and colour as the latrine (e.g. black squares in CF1 represent candidate parental trees of seeds recorded in latrine L1, and red circles in CF2 are candidate parental trees of seeds collected in latrine L3). Black plus (+) symbols in all forest sites represent adults trees for which we did not identified parent-offspring relationships. The remaining forest in (b) is indicated with light grey polygons, the anthropogenic matrix with white, the Lacantún River with light blue, and roads with black lines.
Study primate species
Geoffroy's spider monkey (Ateles geoffroyi Kuhl) is the largest Mesoamerican primate species, and is distributed from the state of Veracruz (Mexico), throughout most Mesoamerica, to northern Colombia. This species is considered a fruit specialist, as ripe fruits account for more than 70% of its feeding time (González-Zamora et al. Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, CHAVES, SÁNCHEZ-LOPEZ, STONER and RIBA-HERNÁNDEZ2009). It has large home range requirements, rapid speed of travel, and a fission-fusion dynamics (Aureli et al. Reference AURELI, SCHAFFNER, BOESCH, BEARDER, CALL, CHAPMAN, CONNOR, DI FIORE, DUNBAR, HENZI, HOLEKAMP, KORSTJENS, LAYTON, LEE, LEHMANN, MANSON, RAMOS-FERNANDEZ, STRIER and VAN SCHAIK2008, Di Fiore & Campbell Reference DI FIORE, CAMPBELL, Campbell, Fuentes, MacKinnon, Panger and Beader2007). Published records of Ateles home range sizes are highly variable (95–900 ha; Wallace Reference WALLACE and Campbell2008). Ateles geoffroyi is probably the primary dispersal agent of S. radlkoferi (Chaves et al. Reference CHAVES, STONER, ARROYO-RODRÍGUEZ and ESTRADA2011, González-Zamora et al. Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, ESCOBAR, RÖS, OYAMA, IBARRA-MANRÍQUEZ, STONER and CHAPMAN2014) as: (1) this tree species is a top food species for spider monkeys throughout its distribution range (González-Zamora et al. Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, CHAVES, SÁNCHEZ-LOPEZ, STONER and RIBA-HERNÁNDEZ2009); and (2) has large diaspores (3–4 cm in length) that are difficult for smaller frugivorous animals to swallow and disperse (Benítez-Malvido et al. Reference BENÍTEZ-MALVIDO, GONZÁLEZ-DI PIERRO, LOMBERA, GUILLÉN and ESTRADA2014, Cramer et al. Reference CRAMER, MESQUITA and WILLIAMSON2007).
Seeds and adult trees of Spondias radlkoferi
The tropical tree Spondias radlkoferi (Anacardiaceae) is self-incompatible and dependent on pollinators (mainly small bees from tribes Meliponini and Exomalopsini) to set its fruit (Carneiro & Martins Reference CARNEIRO and MARTINS2012, Nadia et al. Reference NADIA, MACHADO and LOPES2007). The ranging behaviour of these bees is poorly known, but it has been proposed that when visiting several plants, bees tend to visit first the closest neighbour, or the second nearest neighbour (Collevatti et al. Reference COLLEVATTI, SCHOEREDER and CAMPOS2000, Stacy et al. Reference STACY, HAMRICK, NASON, HUBBELL, FOSTER and CONDIT1996); yet, the nearest-neighbour rule can be violated in fragmented forests (Dick et al. Reference DICK, HARDY, JONES and PETIT2008). It is andromonoecious, i.e. it produces both bisexual and male flowers on the same plant. Although there is no available information on the reproductive phenology of Spondias radlkoferi, S. mombin (a sister and sympatric species) has a synchronous reproductive activity (considering both flowering and fruiting) among populations, being sexually mature at the height of 5–30 m and at an approximate age of 5 y (Adler & Kielpinski Reference ADLER and KIELPINSKI2000). This species (as S. tuberosa) produces many small and white flowers in large inflorescences (Carneiro & Martins Reference CARNEIRO and MARTINS2012, Nadia et al. Reference NADIA, MACHADO and LOPES2007). Anthesis of flowers is sequential within the inflorescence, beginning early morning (c. 05h00), and lasting approximately 2 d to hermaphrodite flowers and only one day to male flowers (Carneiro & Martins Reference CARNEIRO and MARTINS2012, Nadia et al. Reference NADIA, MACHADO and LOPES2007).
Based on recent research on the density and spatial distribution of sleeping trees and latrines of A. geoffroyi in continuous and fragmented forests in the Lacandona region, Mexico (González-Zamora et al. Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, OYAMA, SORK, CHAPMAN and STONER2012, Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, ESCOBAR, RÖS, OYAMA, IBARRA-MANRÍQUEZ, STONER and CHAPMAN2014, Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, ESCOBAR, OYAMA, STONER and AURELI2015), we selected 17 latrines from two forest fragments in MCR (FF1: 1125 ha, 16°15′10.83″N, 90°49′53.82″W; and FF2: 33 ha, 16°16′54.15″N, 90°50′19.91″W) and two continuous forest sites within MABR (CF1: 16°06′25.01″N, 90°59′16.61″W; and CF2: 16°06′08.62″N, 90°58′05.29″W) (Table 1; Figure 1). As latrines we refer to sites beneath sleeping trees where spider monkeys deposit copious amounts of dung (and seeds), and that are repeatedly used (1–12 mo) (González-Zamora et al. Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, OYAMA, SORK, CHAPMAN and STONER2012, Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, ESCOBAR, OYAMA, STONER and AURELI2015). In particular, we selected latrines with at least 25 seeds of S. radlkoferi each (mean = 99 seeds per latrine, range = 25–287 seeds). The average distance among latrines within each forest site varied from 208 to 788 m (Table 1).
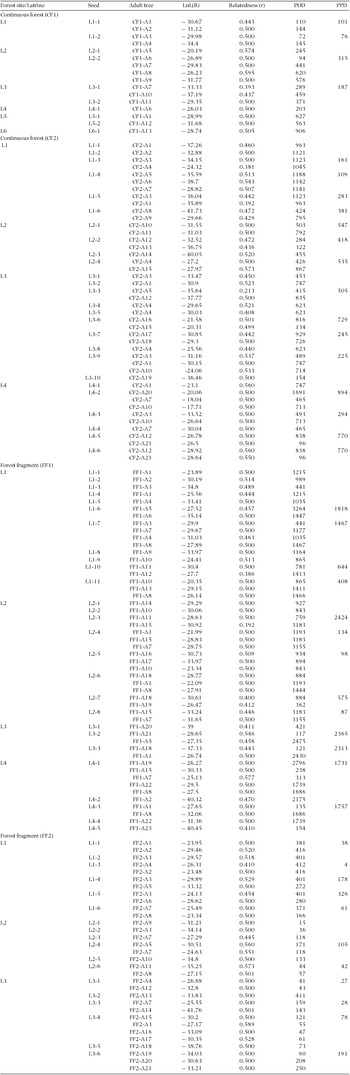
Table 1. Mean parent-parent (PPD, m) and parent-offspring distances (POD, m) of Spondias raldkoferi seeds dispersed by Ateles geoffroyi in latrines located in two continuous forest sites (CF1 and CF2) and two forest fragments (FF1 and FF2) in the Lacandona rain forest, Mexico. The total number of seeds/seedlings, latrines and candidate parent trees included in the analyses are also included, as is the percentage of seeds for which we identified the parental origin. The inter-latrine distance (ILD, m) within each forest site is also indicated.

In the context of a 13-mo study (1 February 2011–28 February 2012) of the seed rain produced by spider monkeys in these latrines, we collected 2880 seeds of S. radlkoferi (see details in González-Zamora et al. Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, ESCOBAR, RÖS, OYAMA, IBARRA-MANRÍQUEZ, STONER and CHAPMAN2014, Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, ESCOBAR, OYAMA, STONER and AURELI2015). The seeds were washed and stored in plastic bags with SilicaGel to avoid the proliferation of fungus and other pathogens. We then randomly selected 1250 undamaged seeds for germination in a greenhouse of the Laboratorio de Ecología Genética y Molecular located at the Universidad Nacional Autónoma de México, Morelia, Mexico. After an average germination period of 120 to 180 d, we collected the leaves of 15 randomly selected seedlings per latrine (total = 255 seedlings) for the genetic analyses that are described below. In the genetic analyses we also included mature leaves of all S. radlkoferi adult trees found within a c. 300-m radius from each latrine. The spatial location of all latrines and adult trees was recorded with a GPS unit (Figure 1).
Microsatellite genotyping
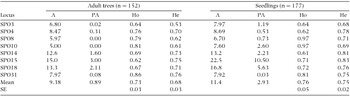
After discarding the individuals for which DNA isolation was not possible, we extracted genomic DNA from 329 plants (152 adult trees and 177 seedlings; Table 1). DNA was isolated from 100 mg of frozen leaf tissue as described in Aguilar-Barajas et al. (Reference AGUILAR-BARAJAS, SORK, GONZÁLEZ-ZAMORA, ROCHA-RAMÍREZ, ARROYO-RODRÍGUEZ and OYAMA2014). We employed eight nuclear microsatellites (SPO3, SPO4, SPO8, SPO10, SPO14, SPO15, SPO18, SPO31) loci previously designed for S. radlkoferi (Aguilar-Barajas et al. Reference AGUILAR-BARAJAS, SORK, GONZÁLEZ-ZAMORA, ROCHA-RAMÍREZ, ARROYO-RODRÍGUEZ and OYAMA2014). As described in Aguilar-Barajas et al. (Reference AGUILAR-BARAJAS, SORK, GONZÁLEZ-ZAMORA, ROCHA-RAMÍREZ, ARROYO-RODRÍGUEZ and OYAMA2014), PCR amplification was performed in a final reaction volume of 5 µl containing 2× QIAGEN multiplex Kit PCR mix (containing HotStar taq DNA polymerase, Multiplex PCR buffer, 3 mM MgCl2 and dNTPs), 0.4 µM of each primer, and ~ 10 ng of DNA template. PCR amplification was performed in Eppendorf Mastercycler (Hamburg, Germany) using the multiplex protocol for amplification of microsatellite loci (QIAGEN Multiplex PCR kit; QIAGEN): first denaturing step 94°C, 15 min; 35 cycles of denaturing 94°C, 30 s; primer annealing at 58°C or 60°C, 1 min 30 s; extension 72°C, 1 min, and finale extension at 72°C for 10 min. Loci that were successfully amplified were then tested with a fluorescent forward primer. Amplified fragments were electrophoresed in an ABI PRISM 3130 XL Genetic Analyzer (Applied Biosystems, Inc., Foster City, CA) with the GeneScan 500 LIZ size standard included (Applied Biosystems, Inc.). We used the PeakScanner software v 1.0 (Applied Biosystems, Inc.) for fragment analysis and final sizing. To detect scoring errors resulting from the presence of null alleles, stuttering or large allele dropout, we tested microsatellite data using the Micro-Checker software (Van Oosterhout et al. Reference VAN OOSTERHOUT, HUTCHINSON, WILLS and SHIPLEY2004).
Genetic diversity
We computed the number of alleles (A) and private alleles (PA) following a rarefaction method that compensates uneven population sizes, as implemented by the HP-Rare software (Kalinowski Reference KALINOWSKI2005). Both the observed (Ho) and expected heterozygosity (He) were determined with the GenAlEx software, version 6.5 (Peakall & Smouse Reference PEAKALL and SMOUSE2006).
Parental analysis and potential pollen- and seed-dispersal distance
Each forest site was analysed separately, i.e. to assess the parental origin of a given seed we considered all adult trees sampled in the forest site where the seed was collected. The genealogical relationship between seeds and adult trees was represented mathematically as probabilities. In particular, we used the ML-RELATE software for parental tests, which uses maximum likelihood to independently identify the most likely relationship category for each pair of individuals in the dataset (i.e. parent-offspring, full sibling, half-sibling, unrelated). The program was designed to accommodate microsatellite loci with null alleles (Kalinowski et al. Reference KALINOWSKI, WAGNER and TAPER2006). For each locus, we tested for the presence of null alleles, as indicated by a deficiency of heterozygotes relative to Hardy–Weinberg expectations. We then applied a correction for the presence of null alleles (Wagner et al. Reference WAGNER, CREEL and KALINOWSKI2006) in the calculations of relatedness (r) and the probability of relationships. Because we have alleles from adult trees and from seedlings, we only evaluated parent–offspring (PO) relationships. We used the specific hypothesis test to determine the parent–offspring pair when the values of likelihood among pedigree relationship were close to each other. In total, we were able to identify the parental origin of 16% and 67% of seedlings tested in CF1 and CF2, and 69% and 50% of seedlings tested in FF1 and FF2, respectively (Table 1). Based on these results, we quantified the PO distances by simply calculating the Euclidian distance between the latrine where the seeds were collected and all candidate parent trees of each seed. For 44 out of 81 seeds (54%) we were able to identify more than one candidate parent tree, which can be considered the maternal and paternal parents of those seeds (Appendix 1). Therefore, we calculated the mean parent-parent (PP) distance per seed, and then the mean PP distance per latrine as a proxy of pollen-dispersal distance. As we cannot distinguish the maternal and paternal parents, we estimated the mean PO distance per seed to have a conservative estimate of potential seed dispersal distances. Although the minimum PO distance would have been an even more conservative estimate of seed dispersal distance than mean PO distance, we selected the latter to avoid underestimations (note that the mean day range of the spider monkey is >2000 m, and this primate can travel up to 4500 m in a day; Wallace Reference WALLACE and Campbell2008).
Statistical analyses
To be conservative in our assessment of potential pollen- and seed-dispersal distances in both forest types (CF and FF), our response variable was a mean of means, i.e. we first calculated the mean PP and PO distance per seed, and then calculated the mean PP and PO distance per latrine (Appendix 1). Although there was no spatial autocorrelation of datasets within the continuous forest sites, nor within the forest fragments (see results of Mantel tests in González-Zamora et al. Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, ESCOBAR, OYAMA, STONER and AURELI2015), we used general linear mixed models to test whether mean PP and PO distances differed between forest types. The fixed effect was forest type, and latrines were nested within each forest site and added as a random effect in the model. The residual maximum likelihood method was used to separate variances of fixed from random effects in the models (Grafen & Hails Reference GRAFEN and HAILS2002). All analyses were done with the JMP 8.0 software.
RESULTS
The loci tested were polymorphic in both parent trees and seedlings (Table 2). Merging the data for CF and FF, parent trees and seedlings showed similar parameters of genetic diversity (Table 2). The mean allelic richness ranged from 5 to 15 in parent trees, and from 7.5 to 22.5 in seedlings. Private alleles were present in both ontogenetic classes, and mean observed and expected heterozygosity was similar in parent trees and seedlings (Table 2). The genetic differentiation between parent trees (FST = 0.05) and seedlings (FST = 0.04) was low.
Table 2. Genetic diversity of the microsatellite loci in adult trees and seedlings of Spondias radlkoferi in the Lacandona region, Mexico. A, allelic richness; PA, private allelic richness; Ho, observed heterozygosity; He, expected heterozygosity.
Global PP and PO distances per latrine averaged 610 m (range = 74–2339 m) and 682 m (range = 83–1741 m), respectively (Appendix 1). Mean PP distance per latrine averaged (± SD) 197 ± 114 m in CF1, 460 ± 186 m in CF2, 1458 ± 737 m in FF1, and 92 ± 26 m in FF2 (Table 1; Figure 1). Mean PO distance averaged 422 ± 288 m in CF1, 570 ± 217 m in CF2, 1436 ± 368 m in FF1, and 204 ± 135 m in FF2 (Table 1; Figure 1). Mean PP distance per latrine did not differ between CF (348 ± 203 m) and FF (872 ± 897 m) (F1,15 = 2.28, P = 0.16; Figure 2a). Similarly, mean PO distance per latrine was statistically similar in CF (524 ± 281 m) and FF (908 ± 714 m) (F1,15 = 2.42, P = 0.14; Figure 2b).
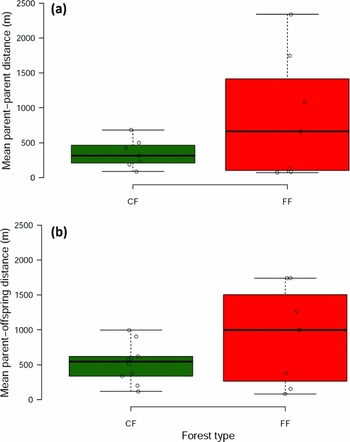
Figure 2. Mean parent-parent (a) and parent-offspring (b) distances in Spondias radlkoferi seeds collected within latrines of spider monkeys located in continuous (CF) and fragmented (FF) forests in the Lacandona rain forest, Mexico. The boxplots indicate the median (thick lines), 1st and 3rd quartiles (box), and the range (whiskers).
DISCUSSION
The ecology of spider monkeys has been investigated for more than five decades (reviewed by Campbell Reference CAMPBELL2008). However, this study is the first to assess the parental origin of seeds dispersed by the spider monkey in a fragmented rain forest using molecular methods. Our findings suggest that Spondias radlkoferi seeds deposited by spider monkeys in latrines came from distantly located parents, as PO distances per latrine averaged 908 m in forest fragments and 524 m in continuous forests. Although we cannot distinguish between maternal and paternal parents, our results suggest that long-dispersal distances (>100 m) are common in both forest types, as 87–93% of estimate values of mean PO distance per seed were higher than 100 m in forest fragments and continuous forest sites, respectively (Appendix 1). In agreement with previous reports for other tropical trees (reviewed by Dick et al. Reference DICK, HARDY, JONES and PETIT2008), our findings also suggest that pollen dispersal is extensive in both forest types as mean PP distances per latrine averaged 347 m in continuous forest sites and 872 m in forest fragments.
Our results support the idea that spider monkeys (Ateles spp.) are effective seed dispersers (Chaves et al. Reference CHAVES, STONER, ARROYO-RODRÍGUEZ and ESTRADA2011, Dew Reference DEW and Campbell2008, González-Zamora et al. Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, ESCOBAR, RÖS, OYAMA, IBARRA-MANRÍQUEZ, STONER and CHAPMAN2014, Link & Di Fiore Reference LINK and DI FIORE2006). Ateles geoffroyi, in particular, has a very high dietetic diversity throughout its geographic range (364 species, 76 families), and fruits are the most common food item in their diet (González-Zamora et al. Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, CHAVES, SÁNCHEZ-LOPEZ, STONER and RIBA-HERNÁNDEZ2009). In fact, they are considered ripe fruit specialists (Di Fiore & Campbell Reference DI FIORE, CAMPBELL, Campbell, Fuentes, MacKinnon, Panger and Beader2007). In the Lacandona rain forest, A. geoffroyi feeds on 73 fruit species and swallow seeds of most of them, thus promoting that most spider monkey faeces contain seeds (Chaves et al. Reference CHAVES, STONER, ARROYO-RODRÍGUEZ and ESTRADA2011). In fact, González-Zamora et al. (Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, ESCOBAR, RÖS, OYAMA, IBARRA-MANRÍQUEZ, STONER and CHAPMAN2014) demonstrate that spider monkeys deposited > 45 000 seeds (> 5 mm in length) from 68 plant species in 60 latrines during a 13-mo period. Thus, considering the quantitative component of seed dispersal effectiveness (sensu Schupp Reference SCHUPP1993), which depends on the number of seeds that are dispersed, there is no doubt that the spider monkey is an effective seed disperser.
Regarding the quality component of seed dispersal effectiveness, which depends on the quality of treatment given to the seed in the animal's mouth and gut, and on the quality of seed deposition (Schupp Reference SCHUPP1993), there is also evidence that spider monkeys are effective seed dispersers. For example, although there is evidence of positive, negative and neutral net effects of primate gut passage on seed germination, positive effects are more frequent (reviewed by Chaves et al. Reference CHAVES, STONER, ARROYO-RODRÍGUEZ and ESTRADA2011). The number of S. radlkoferi seeds that successfully germinate is actually higher for defecated seeds than for control (i.e. from mature fruits) seeds (Chaves et al. Reference CHAVES, STONER, ARROYO-RODRÍGUEZ and ESTRADA2011). Also, seed deposition patterns produced by spider monkeys are mixed, i.e. a fraction clumped in latrines and another one scattered across the forest (Chaves et al. Reference CHAVES, STONER, ARROYO-RODRÍGUEZ and ESTRADA2011, González-Zamora et al. Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, ESCOBAR, RÖS, OYAMA, IBARRA-MANRÍQUEZ, STONER and CHAPMAN2014, Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, ESCOBAR, OYAMA, STONER and AURELI2015; Russo & Augspurger Reference RUSSO and AUGSPURGER2004). This contributes to creating heterogeneous seed deposition patterns, which can favour the colonization of suitable sites for seedling recruitment (Howe & Smallwood Reference HOWE and SMALLWOOD1982).
The present study adds further evidence on the effectiveness of A. geoffroyi as a seed disperser, as we demonstrate that mean PO distance per latrine was highly variable (83–1741 m), but most figures were far above 100 m – a typical threshold for long dispersal distances (sensu Cain et al. Reference CAIN, MILLIGAN and STRAND2000) (Figure 1, 2; Appendix 1). The fact that we identified putative parental trees for only 16–69% of seeds sampled in each forest site can actually be interpreted as a potential underestimation of PO distances (i.e. the parents of 31–84% of seeds would be located outside of the sampled area). In any case, our numbers in both continuous and fragmented forests were within the range values reported for A. belzebuth in the Yasuni National Park, Ecuador (up to 1000 m; Dew Reference DEW and Campbell2008, Link & Di Fiore Reference LINK and DI FIORE2006). Therefore, seed dispersal by spider monkeys can be of key relevance for seedling recruitment and survival, as long dispersal distance allows seeds and seedlings escaping from areas of high mortality close to parental trees (Howe & Smallwood Reference HOWE and SMALLWOOD1982).
Although we cannot directly measure seed dispersal distances, we are confident that our estimations of mean PO distances can be considered a conservative proxy of seed dispersal distances. First, our figures were within the range reported for other Ateles species. Second, they are consistent with our knowledge about the ranging behaviour and gut passage times in spider monkeys. In particular, records of mean day journey length (i.e. day range) are variable, but most mean estimates fall between 2000 and 2300 m (reviewed by Wallace Reference WALLACE and Campbell2008). Thus, because gut passage times range from c. 2.5 to 18 h (Milton Reference MILTON1981, Russo et al. Reference RUSSO, PORTNOY and AUGSPURGER2006), we can expect that seed dispersal distances rarely exceed 2000 m, if they do. Finally, as our estimations included all PO relationships (including both maternal and paternal parents) per seed, and we averaged PO distances per latrine (i.e. considering all seeds recorded within each latrine), it is reasonable to suppose that the PO distances reported in this study represent conservative estimation of potential seed dispersal distances per latrine.
Regarding our estimations of parent-parent distances, our findings support the idea that pollinators usually violate the nearest-neighbour rule (Dick et al. Reference DICK, HARDY, JONES and PETIT2008). Although the ranging behaviour of the primary pollinators of S. radlkoferi (e.g. Meliponini and Exomalopsini bees) is largely unknown, our findings support the idea that they travel long distances (> 2 km) in search of flowers. This figure is not surprising, as there is increasing evidence of long pollen-dispersal distances in temperate and tropical trees, including trees pollinated by very small insects, such as figs (Ficus spp.), which can show pollen-dispersal distances of > 5 km (reviewed by Dick et al. Reference DICK, HARDY, JONES and PETIT2008). This suggests that, as in other highly outcrossed mating systems, extensive long-distance pollen dispersal probably plays a key role in maintaining the genetic diversity of S. radlkoferi, thereby limiting potential negative genetic effects of inbreeding and drift in fragmented populations (Millar et al. Reference MILLAR, COATES and BYRNE2014). Yet, additional studies with precise paternity assignments (i.e. paternal parent), and those capable of tying dispersed seeds back to the particular tree that bore the fruit consumed by the monkeys (i.e. maternal parent) are needed to accurately quantify pollen- and seed-dispersal distances (Dick et al. Reference DICK, HARDY, JONES and PETIT2008, Godoy & Jordano Reference GODOY and JORDANO2001, Grivet et al. Reference GRIVET, SMOUSE and SORK2005).
Ecological implications
Although contagious seed dispersal (e.g. in primate latrines) can reduce the quality of dispersal because it leads to dispersal and recruitment limitation (Schupp et al. Reference SCHUPP, MILLERON, RUSSO, Levey, Silva and Galetti2002), an increasing number of studies suggest that primate latrines can be of key relevance for forest regeneration (Arroyo-Rodríguez et al. Reference ARROYO-RODRÍGUEZ, ANDRESEN, BRAVO, STEVENSON, Kowalewski, Garber, Cortés-Ortiz, Urbani and Youlatos2015, González-Zamora et al. Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, OYAMA, SORK, CHAPMAN and STONER2012, Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, ESCOBAR, RÖS, OYAMA, IBARRA-MANRÍQUEZ, STONER and CHAPMAN2014, Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, ESCOBAR, OYAMA, STONER and AURELI2015; Russo & Chapman Reference RUSSO, CHAPMAN, Campbell, Fuentes, MacKinnon, Panger and Bearders2011). Considering spider monkey latrines, for instance, evidence indicates that they are relatively abundant in the forests: up to 0.16 latrines ha−1 in the Calakmul region, south-eastern Mexico (Velázquez-Vázquez et al. Reference VELÁZQUEZ-VÁZQUEZ, REYNA-HURTADO, ARROYO-RODRÍGUEZ, CALMÉ, LÉGER-DALCOURT and NAVARRETE2015), and 0.53 latrines ha−1 in the Lacandona rain forest (González-Zamora et al. Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, OYAMA, SORK, CHAPMAN and STONER2012). The spatial distribution of spider monkey latrines is highly variable across the forest (González-Zamora et al. Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, OYAMA, SORK, CHAPMAN and STONER2012), thus increasing the probability that some latrines are located in suitable sites for seed germination and seedling recruitment. The accumulation of copious amounts of faeces in latrines results in soil nutrient enrichment, which further favour the establishment, growth and survival of seedlings (reviewed by Arroyo-Rodríguez et al. Reference ARROYO-RODRÍGUEZ, ANDRESEN, BRAVO, STEVENSON, Kowalewski, Garber, Cortés-Ortiz, Urbani and Youlatos2015). Seedling recruitment can also be favoured by the fact that the very high seed arrival rate and high seed germination rates in primate latrines can lead to the saturation of biotic mortality agents (e.g. seed/seedling predators) (González-Zamora et al. Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, ESCOBAR, RÖS, OYAMA, IBARRA-MANRÍQUEZ, STONER and CHAPMAN2014, Russo & Augspurger Reference RUSSO and AUGSPURGER2004), particularly in latrines that are more frequently used by the monkeys, which receive more seeds and from a higher number of plant species (González-Zamora et al. Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, ESCOBAR, OYAMA, STONER and AURELI2015).
Here we present additional evidence on the importance of latrines for seedling recruitment and forest regeneration, as we found that seeds deposited in spider monkey latrines came from different distant places. This pattern can be related to the fruiting pattern of S. radlkoferi, and the fission-fusion dynamics of spider monkeys (Aureli et al. Reference AURELI, SCHAFFNER, BOESCH, BEARDER, CALL, CHAPMAN, CONNOR, DI FIORE, DUNBAR, HENZI, HOLEKAMP, KORSTJENS, LAYTON, LEE, LEHMANN, MANSON, RAMOS-FERNANDEZ, STRIER and VAN SCHAIK2008), and their complex feeding and ranging behaviours (Ramos-Fernández et al. Reference RAMOS-FERNÁNDEZ, SMITH-AGUILAR, SCHAFFNER, VICK and AURELI2013). In particular, fruiting is spatially and temporally aggregated, thus allowing monkeys to visit several adult trees near their sleeping trees (Russo et al. Reference RUSSO, PORTNOY and AUGSPURGER2006). In fact, the probability of visiting different S. radlkoferi adult trees can be promoted by the fact that spider monkeys use home ranges and core areas of variable sizes through time and space (Ramos-Fernández et al. Reference RAMOS-FERNÁNDEZ, SMITH-AGUILAR, SCHAFFNER, VICK and AURELI2013), and also, by the fact that groups are split in subgroups of different sizes and composition during the day to feed on several plants located near sleeping trees, and return to the same or different sleeping trees after foraging excursions (Aureli et al. Reference AURELI, SCHAFFNER, BOESCH, BEARDER, CALL, CHAPMAN, CONNOR, DI FIORE, DUNBAR, HENZI, HOLEKAMP, KORSTJENS, LAYTON, LEE, LEHMANN, MANSON, RAMOS-FERNANDEZ, STRIER and VAN SCHAIK2008). As argued by Terakawa et al. (Reference TERAKAWA, ISAGI, MATSUI and YUMOTO2009) for the Yakushima macaque, the fact that monkey faeces contain seeds from different distantly located parent trees increases the fitness of parental trees by limiting intra-sibship competition and inbreeding depression. Also, and perhaps more importantly, seed germination and seedling recruitment in latrines may be favoured by the fact that seeds from different parents have different genotypes, and hence, different abilities to cope with the heterogeneous environmental conditions that are present in latrines (Jordano Reference JORDANO, Dennis, Green, Schupp and Wescott2007, Terakawa et al. Reference TERAKAWA, ISAGI, MATSUI and YUMOTO2009). Nevertheless, additional studies will be required to accurately assess seed germination and seedling recruitment in spider monkey latrines, as there exist very few studies on this topic, and hence, our understanding on the role that primate latrines may have on forest regeneration is still limited (Arroyo-Rodríguez et al. Reference ARROYO-RODRÍGUEZ, ANDRESEN, BRAVO, STEVENSON, Kowalewski, Garber, Cortés-Ortiz, Urbani and Youlatos2015, González-Zamora et al. Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, ESCOBAR, RÖS, OYAMA, IBARRA-MANRÍQUEZ, STONER and CHAPMAN2014, Reference GONZÁLEZ-ZAMORA, ARROYO-RODRÍGUEZ, ESCOBAR, OYAMA, STONER and AURELI2015; Russo & Chapman Reference RUSSO, CHAPMAN, Campbell, Fuentes, MacKinnon, Panger and Bearders2011).
ACKNOWLEDGEMENTS
E.A.B. is a postdoctoral student, supported by DGAPA–UNAM. The Instituto de Investigaciones en Ecosistemas y Sustentabilidad, UNAM, the Escuela Nacional de Estudios Superiores, UNAM, and the Instituto de Investigaciones Biológicas, UV, provided logistical support. AGZ thanks the scholarship (Apoyos Complementarios para la Consolidación Institucional de Grupos de Investigación) provided by CONACyT. This study would not have been possible without the collaboration of Natura y Ecosistemas Mexicanos A.C. and the local people in Chajul, Reforma Agraria, and Zamora Pico de Oro ejidos. We thank R. Lombera for invaluable field assistance and Jake C. Dunn for his helpful suggestions on the manuscript. H. Ferreira, A. Valencia and A. López provided technical support. The research methods adhered to Mexican legal requirements and animal care regulations.
Appendix 1. Parent-parent and parent-offspring relationships in Spondias radlkoferi trees in two continuous and two fragmented forest sites in the Lacandona rain forest, Mexico. We indicate the log-likelihood and relatedness coefficient of each parent (adult tree)-offspring (seed) relationship, the Euclidian distance between each seed (collected in latrines of spider monkeys) and each candidate parental tree (i.e. parent-offspring distance, POD, m), and for those cases in which we found more than one candidate parent for a given seed, we also show parent-parent distances (PPD, m).