Environmental cleaning and disinfection of clinical surfaces are essential for infection prevention programs to be effective.Reference Goodman, Platt, Bass, Onderdonk, Yokoe and Huang1,Reference Dancer2 There is now a strong evidence of the role played by contaminated healthcare surfaces in the transmission of pathogens that cause healthcare-associated infections, including Clostridium difficile, methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococci (VRE), norovirus, and multidrug-resistant (MDR) gram-negative bacteria including Acinetobacter baumannii. These pathogens have been shown to survive on surfaces for prolonged periods (days, months, and even years) and to remain virulent.Reference Weber, Rutala, Miller, Huslage and Sickbert-Bennett3 Their transmission can occur directly or through secondary vehicles such as hands.Reference Weber, Rutala, Miller, Huslage and Sickbert-Bennett3
Recent studies have highlighted the evidence that current cleaning and disinfection practices might not be effectiveReference Doll, Stevens and Bearman4 despite good adherence to infection control guidelines.Reference Holmes, Castro-Sánchez and Ahmad5,Reference Iwami, Ahmad, Castro-Sánchez, Birgand, Johnson and Holmes6 One possible explanation for this failure encountered in practice is the presence of bacteria in complex dry-surface biofilms (DSBs).Reference Hu, Johani and Gosbell7–Reference Yezli and Otter9 Multidrug-resistant bacteria have been shown to be present in DSBs on bedding, surrounds, and furnishing items taken from intensive care units, despite cleaning with chlorine solution.Reference Vickery, Deva, Jacombs, Allan, Valente and Gosbell10
Internationally accepted standardized protocols to test disinfectants and detergent product effectiveness against “hydrated” biofilms are still lacking,Reference Almatroudi, Hu and Deva11 let alone DSBs. In addition, most standard efficacy tests investigate a reduction in microbial viability, but rarely do they consider microbial transfer after treatment or the ability of biofilm to reform rapidly after treatment.Reference Schultz, Bjarnsholt and James12,Reference Han, Song and Zhang13 DSBs have been shown to be transferred via hands to multiple fomites,Reference Chowdhury, Tahir and Legge14 and the implications of this transfer in pathogen transmission has been identified.Reference Han, Sullivan, Leas, Pegues, Kaczmarek and Umscheid15 The role of gloves as DSB transfer vehicles has been also recognized.Reference Chowdhury, Tahir and Legge14 In addition, DSBs can recover even following very strong chlorine treatment (ie, 20,000 ppm).Reference Almatroudi, Gosbell and Hu8
In this study, we investigated the combined reduction in viability, transferability, and recovery of bacteria from DSBs following treatments with 11 commercially available disinfectants, 4 detergents, and 2 contactless interventions. We believe that these 3 factors combined provide a full picture of product effectiveness against DSBs, and hopefully, this information will help in the development of improved infection control guidelines for routine and terminal cleaning of hospital surfaces.
Methods
Bacterial growth and maintenance
Staphylococcus aureus NCTC10788 was propagated in tryptone soya broth (TSB; Oxoid, Hampshire, UK) at 37 °C overnight and was washed in tryptone sodium chloride [1 g/L of tryptone; Oxoid, Fisher Scientific, Loughborough, UK) and 8.5 g/L of sodium chloride (Sigma-Aldrich, Dorset, UK)] following centrifugation at 1,400×g. The bacterial suspension was adjusted to 1×106 CFU/mL.
Products tested
In total, 15 commercially available products were tested; they are summarized in Table 1.
Table 1. Disinfectants and detergents tested
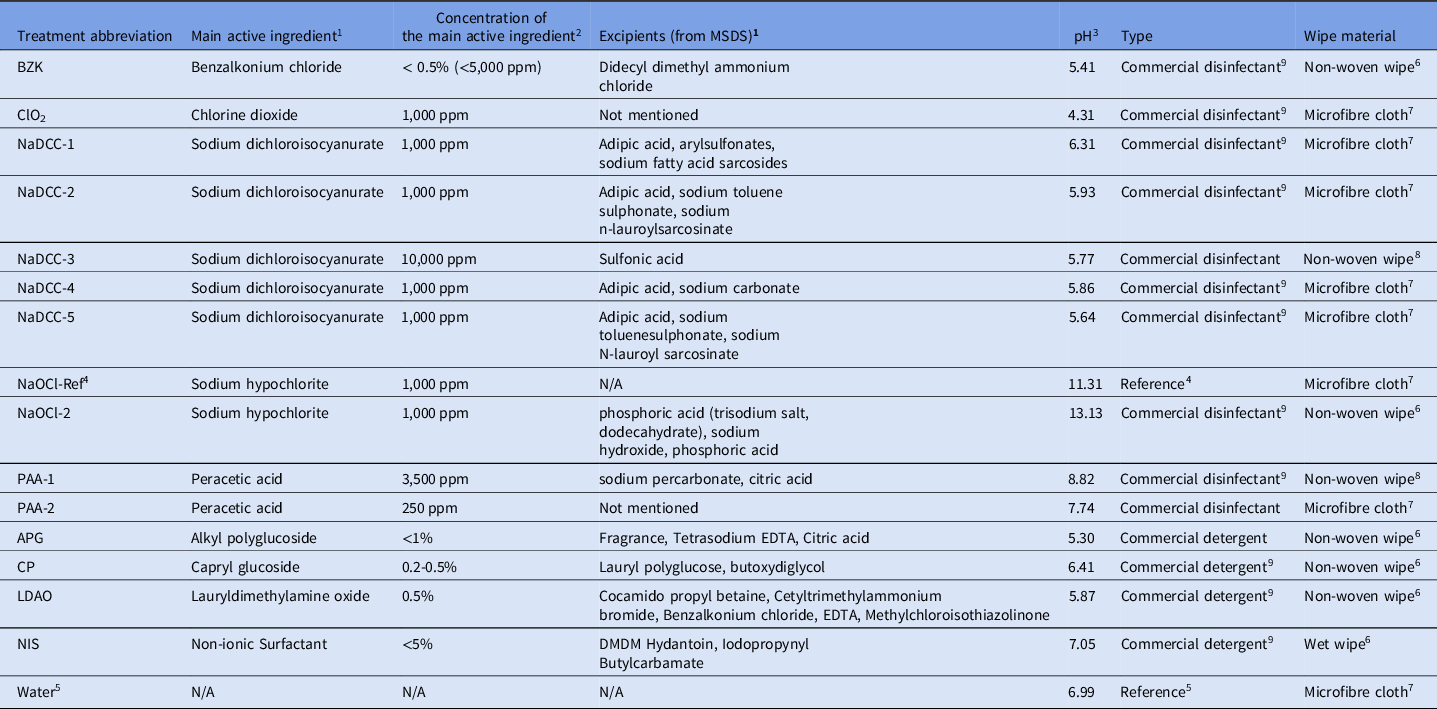
1 Main active ingredient and excipients mentioned in the MSDS information of the commercial products used in this study.
2 Concentration of available chlorine was measured with Pocket Colorimeter™ (HACH®, Manchester, UK) (regardless of the product claim on label).
3 pH was measured by FiveEasy Standard pH Meter (Mettler-Toledo Ltd., Leicester, UK).
4 Unformulated sodium hypochlorite (1,000 ppm) used as reference.
5 Sterile deionized water.
6 Wipe originally moisturized with disinfectant by the manufacturer.
7 Disinfectant prepared according to manufacturer’s instruction and then placed on Rubbermaid® HYGEN™ disposable microfiber cloth (Rubbermaid Products, Surrey, UK) 2.5 ml of liquid per 1 g of cloth.
8 Dry non-woven wipe impregnated with powder particles - needs to be wetted according to manufacturer instructions prior to use.
9 Available in National Health Service (NHS) Supply Chain.
Neutralizer preparations
The universal neutralizer was prepared according to a modified protocol.Reference Holah, Taylor, Dawson and Hall16 Briefly, lecithin 3 g/L (ACROS Organics, Fisher Scientific), Tween80 30 g/L (ACROS Organics, Fisher Scientific), sodium thiosulphate 5 g/L (Fisher Bioreagents, Fisher Scientific), L-histidine 1 g/L (Sigma-Aldrich), saponin 30 g/L (ACROS Organics, Fisher Scientific), and sodium dodecyl sulphate 5 g/L (Sigma-Aldrich) in sterile tryptone sodium chloride solution were mixed together. Distilled water was added to a volume of 1 L, and the solution was then autoclaved at 121 °C for 15 minutes.
DSB formation
We grew DSB following the methods of Ledwoch et al.Reference Ledwoch, Said, Norville and Maillard17 Briefly, S. aureus NCTC 10788 overnight culture was centrifuged at 1,400×g in a Biofuge Primo R centrifuge (Heraeus, Thermo Fisher Scientific, Newport, UK) and washed with fresh tryptone soya broth (TSB; Oxoid, Thermo Fisher Scientific). The S. aureus culture was then diluted to 106 CFU/mL in the presence of 3 g/L bovine serum albumin (BSA, Sigma Life Science, Dorset, UK). The culture was inoculated onto stainless steel AISI 430 discs (0.7 ± 0.07 mm thickness; 10 ± 0.5 mm diameter, Goodfellow Cambridge, Huntington, UK) by transferring 1 mL inoculum and a single disc to each well of the Corning Costar flat-bottom cell-culture plates (Fisher Scientific). The well plate containing discs and inoculum was then placed on an Orbit P4 plate rocker (Labnet International, Edison, NJ) at 180 rpm and 21 °C for 2 days (the so-called “wet phase”). Following the wet phase, the liquid was drained from the wells and the well plate was moved into a universal oven 100-800 incubator (Memmert, Schwabach, Germany) at 37 °C for 2 days (the so-called “dry phase”). After the dry phase, 1 mL TSB with 3 g/L BSA was added to each well to begin another wet phase. The biofilms were grown in alternating wet–dry phases for a total duration of 12 days.
ASTM E2967-15 Wiperator test
The effectiveness of commercial products commonly used in hospitals was investigated according to a modified ASTM E2967 test.18 All products were tested in a wipe form except VHP and CAP. If the product was supplied in a liquid form, it was combined with sterile Rubbermaid HYGEN disposable microfiber cloth (Rubbermaid, Surrey, UK), allowing 2.5 mL disinfectant per 1 g wipe. The surface of the disc with the DSB was wiped with the Wiperator (Filtaflex, Ontario, Canada) from both sides using separate wipes. DSBs were wiped for 10 seconds under 500 g pressure and were left at room temperature for 2 minutes (contact time). Transfer of viable bacteria from used wipes to clean sterile discs was not performed.
Vaporized hydrogen peroxide test
Vaporized hydrogen peroxide (VHP) testing was performed at GAMA Healthcare (Watford, UK) with a RHEA VHP device (Airinspace, Élancourt, France). The biocide used in this system consisted of 7% of hydrogen peroxide, 5% acetic acid, and 0.4% peracetic acid. Briefly, S. aureus DSB samples were placed on a stand at 1.5 m height and were arranged vertically 3 m away from VHP unit. The treatment ran for 90 minutes with 6 mL biocide used per cubic meter of the room. In total, 290 mL biocide was consumed per cycle. Following treatment, samples were neutralized by placing each disc in separate well containing 1 mL Dey-Engley (DE) neutralizing broth (Neogen, Ayr, UK) for 24 hours.
Cold atmospheric plasma treatment
Cold atmospheric plasma (CAP) treatment was performed using a surface barrier discharge device developed at the Centre for Plasma Microbiology, Department of Electrical Engineering and Electronics, University of Liverpool.Reference Modic, McLeod, Sutton and Walsh19 The distance between the S. aureus DSB disc and the plasma-generating electrode was 2 mm, providing a spatial separation between the plasma layer and the sample surface. Coupons were exposed to an reactive nitrogen species (RNS)–dominated gas-phase chemistry created using a high-power discharge plasma (Pdischarge = 34.5 W). Treatments were performed for 240 seconds. Discs were treated from both sides. Following treatment, the samples were neutralized by placing each disc in separate well containing 1 mL DE neutralizing broth for 24 hours.
Reduction in bacterial viability
Reduction in bacterial viability (log10 reduction in CFU per mL) gave the number of bacteria that were removed and/or killed following wiping. The test was performed following the methods of Ledwoch et al.Reference Ledwoch, Said, Norville and Maillard17 Briefly, following 2 minutes of contact time (or VHP/CAP treatment), the discs were neutralized by placing each disc into universal neutralizer for 5 minutes.
Following neutralization, the discs were moved to McCartney bottles containing 2 mL TSB with 1 g glass beads and were incubated for 2 hours at 37 °C. After incubation, the bottles with discs were spun in a vortex mixer for 2 minutes to remove DSB from the disc surface, and the solution was then plated by dropping it onto tryptone soya agar (TSA; Oxoid, Thermo Fisher Scientific). The log10 reduction was evaluated against controls consisting of untreated samples. The reduction in viability was measured for disinfectants only.
Direct transfer test
The surface transferability test was designed to investigate how much bacteria can be transferred directly from treated surface. A direct transfer test was performed following the methods of Ledwoch et al.Reference Ledwoch, Said, Norville and Maillard17 Following wiping and 2-minute contact time, or VHP and CAP treatment, each disc was picked up by an Alnico Rod Magnet (Rapid Electronics, Essex, UK) and pressed 36 consecutive times with 100 g pressure on the surface of Dey-Engley (DE) neutralizing agar (Sigma-Aldrich) (Supplementary Fig. S1 online, left). Following the transfer test, DE agar was incubated overnight at 37 °C. Positive growth divided by the total number of compressions was recorded. Direct transferability was measured for disinfectants only.
Cross transmission via glove (glove transferability)
The test imitates the single touch of a gloved finger onto a treated surface and subsequent transfers from the contaminated glove to the environment. Following wiping and a 2-minute contact time, disc was pressed once with 100 g pressure by a gloved finger (Supplementary Fig. S1 online, right). Then, the gloved finger was pressed 25 consecutive times with 100 g pressure on the surface of Dey-Engley (DE) neutralizing agar (Sigma-Aldrich). Following the transfer test, DE agar was incubated overnight at 37 °C. Positive growth was recorded, and transferability was expressed as the number of positive contact divided by the total number of compressions (Supplementary Fig. S3 online). Cross transmission was measured for both disinfectant and detergent products. Two different glove types were tested: ecoSHIELD powder-free nitrile gloves (Appleton Woods, Birmingham, UK) and SemperGuard 100% latex-powder–free inner-coated gloves (Appleton Woods).
Regrowth test
Regrowth measures the time needed for the DSB to recover following treatment. The regrowth testing was performed following the methods of Ledwoch et al.Reference Ledwoch, Said, Norville and Maillard17 Following wiping and a 2-minute contact time, samples were placed in a 30-mL flat-bottom glass bottle with 2 mL Dey-Engley (DE) neutralizing broth (Neogen). The number of days for the DE neutralizing broth color to change from purple to yellow (indicative of bacterial growth) was recorded. Regrowth was measured for disinfectants only.
Pass/fail criteria
The ASTM E2967 protocol does not have any pass or fail criteria. Here, we arbitrarily set it to >4 log10 to reflect the minimal reduction requirement indicated in European standards.20 We also suggested that an effective biocidal treatment would reduce bacterial transferability to a maximum of 2 positive contacts.
The UK National Health Service specifies a 0–48-hour period for cleaning low-risk functional areas.21 Due to the potentially low cleaning compliance in hospitals and because some surfaces are overlooked in routine cleaning,Reference Ramphal, Suzuki, McCracken and Addai22 we suggested that successful treatment must prevent biofilm regrowth for at least 3 days.
Statistical analysis
The statistical significance of data sets was evaluated with PRISM version 7.04 software (GraphPad, San Diego, CA) using single-factor analysis of variance (ANOVA). All measurements were performed in triplicate. The sample standard deviation was evaluated using the Bassel correction.
Results
Reduction in viability
Overall, most disinfectants tested passed the S. aureus DSB viability reduction test (Table 2), and 6 disinfectants performed particularly well, achieving ≥6 log10 reduction: 4 NaDCC and 2 PAA formulations (Table 2). ClO2 (1,000 ppm; pH = 4.31) and NaOCl2 (1,000 ppm; pH = 13.13) were the least effective treatments. There was no statistically significant difference between the performance of ClO2 and NaOCl2 treatments and just wiping with water (ANOVA: single factor, P = .06 for ClO2 and P = .14 for NaOCl2). This result was surprising given that both ClO2 and NaOCl2 products have the same level of available chlorine (ie, 1,000 ppm) as the other chlorine-based products that achieved a >6 log10 reduction. However, the relation between pH of tested disinfectant and the log10 reduction in DSB viability showed that underperforming products had extreme pH levels (ClO2 pH = 4.31 and NaOCl2 pH = 13.13) compared to the rest of the products tested. Biocides formulated at pH ~8 were the most effective among the tested products (Fig. 1). Both VHP and CAP interventions that did not involve mechanical wiping performed poorly (<1 log10 reduction) against DSBs (Table 2).
Table 2. The effectiveness of various treatments in bacterial viability reduction (log10 reduction), bacterial transferability reduction (direct and cross transferability) and recovery prevention (regrowth) of artificial S. aureus NCTC10788 dry surface biofilm formed in accordance with Ledwoch et al. (17). For pass/fail criteria see text
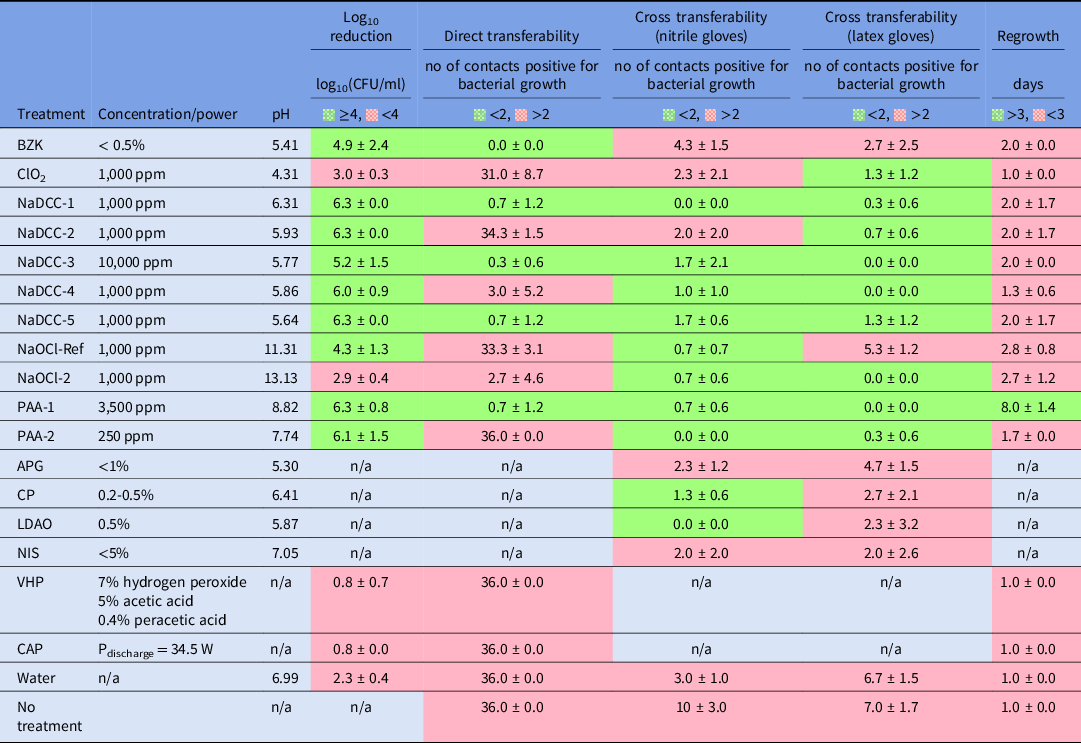
n/a- not applicable/ not tested.
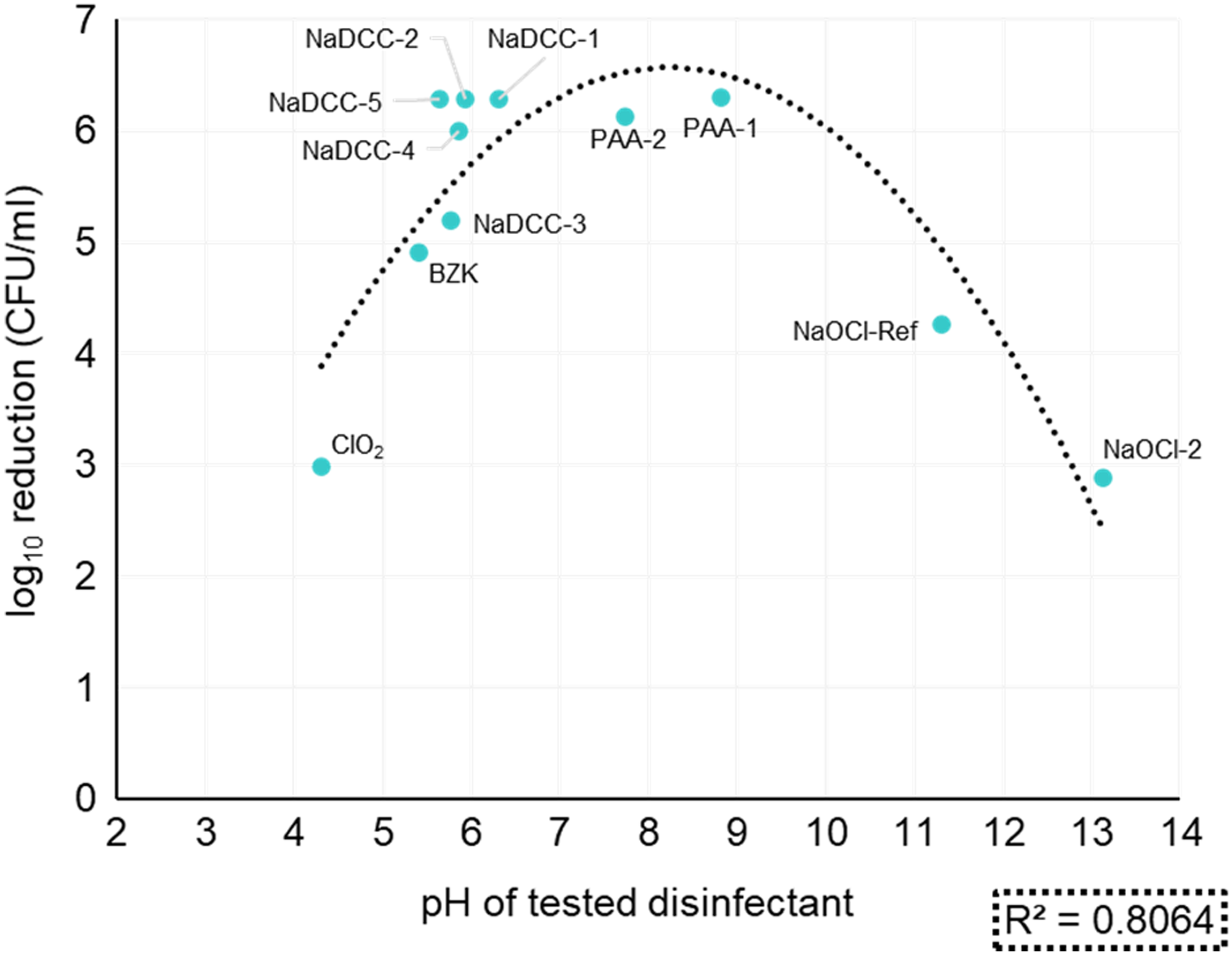
Fig. 1. Log10 reduction of S. aureus DSB in relation to the pH of tested disinfectant.
Direct transfer (surface transferability)
Only 5 biocides (BZK, NaDCC-1, NaDCC-3, NaDCC-5, and PAA-1) were successful at preventing direct bacterial transfer from DSBs (Table 2). There was no difference in bacterial transfer (ie, 100% transfer; Supplementary Fig. S2 online, right panel) between wiping the surface with just water and applying ClO2, NaDCC-2, NaOCl-Ref, PAA-2, VHP or CAP treatments (ANOVA: single factor, P > .05).
Cross transmission (glove transferability)
Both peracetic acid treatments successfully controlled the transmission of bacteria via nitrile and latex gloves. All chlorine-based formulated disinfectants were effective at preventing the transferability of bacteria from DSB via latex glove. Most of the chlorine-based products were also successful when the nitrile glove was used. However, some treatments failed to control bacterial transferability despite the high reduction in bacterial viability reduction, as has been shown in previous experiments. The inverse phenomenon was observed with NaOCl-2, when bacteria were not transferred from the DSB, even though the treatment was not effective at significantly lowering biofilm viability.
Bacterial regrowth
Only 1 treatment, PAA-1, prevented the recovery of bacteria for >3 days (Table 2). The recovery of biofilm was observed within 2 days for the vast most of the treatments, even when their biofilm viability reduction or transferability prevention was satisfactory.
Discussion
It has now been well established that environmental surfaces in healthcare settings act as reservoirs for pathogens that contribute to their transmission.Reference Otter, Yezli, Salkeld and French23
Here, we have argued that current practices investigating the effectiveness of environmental surfaces disinfection are insufficient. Efficacy surface tests are based on planktonic bacteria dried onto surfaces (eg, ASTM E2967), and they do not reflect nor mimic biofilmsReference Tahir, Chowdhury and Legge24 that are actually present on hospital surfaces.Reference Hu, Johani and Gosbell7,Reference Almatroudi, Gosbell and Hu8,Reference Ledwoch, Dancer and Otter25 The S. aureus used in our model is a well-known pathogen that can survive desiccation, and its presence in DSBs is widespread.Reference Hu, Johani and Gosbell7,Reference Ledwoch, Dancer and Otter25 Moreover, wiping mechanical action is thought to contribute to biofilm removal with disruption of the extracellular polymeric substance (EPS) matrix, weakening its effectiveness as a protective barrier to disinfection. We combined our S. aureus DSB model with the ASTM E2967 product test with a contact time of 2 minutes, which is considered more realistic in practice.Reference Siani and Maillard26 This test acknowledges the importance of mechanical wiping in evaluating the efficacy of products.Reference Sattar and Maillard27 Here, nonmechanical treatments tested (CAP and VHP) showed little activity (<1 log10 reduction) against S. aureus DSB (Table 2), with no decrease in bacterial transfer or delay in biofilm regrowth (Table 2). In contrast, wiping with water produced a 2.3 ± 0.4 log10 reduction in viability (Table 2). The nature of DSB might also have affected the lack of efficacy of CAP. Indeed, a ‘wet’ S. aureus ATCC 9144 biofilm has been completely eradicated following CAP treatment for 1 minute.Reference Modic, McLeod, Sutton and Walsh19 Where wiping was used, 9 of the 11 disinfectants tested decreased S. aureus viability by >4 log10, which was used as a criterion for efficacy.Reference Wesgate, Robertson, Barrell, Teska and Maillard28 We have highlighted that a formulation’s pH might be a factor that contributes to efficacy (Fig. 1) because bioactive compounds formed outside their optimum pH range may be less stable.Reference Chapter29
We have argued that measuring the biocidal efficacy of a formulation might not be solely indicative of product performance, particularly when biofilm eradication is studied. Indeed, when combining efficacy data with impact of treatment on bacterial transfer direct or via gloves, a different picture emerges (Table 2). Only formulated PAA (PAA-1, 3,500 ppm) achieved all set criteria, combining efficacy, decreasing direct and indirect transfer, and preventing biofilm regrowth.
The meaning of “rendering a surface safe” can be debated. However, it is appropriate to reflect on the role of bacterial transmission with gloved hands. The role of gloves to transfer pathogens has been reportedReference Tahir, Chowdhury and Legge24,Reference Moore, Dunnill and Wilson30 with latex and nitrile gloves when bacteria were suspended in tryptone soya broth + 5% horse serum.Reference Moore, Dunnill and Wilson30 Treatment with detergent has been reported to increase the transferability of bacteria from DSBs.Reference Chowdhury, Tahir and Legge14 However, we have shown that most interventions reduced bacterial transfer from surfaces to gloves (Table 2).
Bacteria in a biofilm surviving a biocidal treatment can initiate the formation of a new biofilm.Reference Han, Song and Zhang13 Indeed, studying biofilm regrowth after treatment not only provides important information regarding product efficacy but also indicates the frequency of product application to ensure that surfaces remain safe.Reference Ledwoch, Dancer and Otter25 Biofilms have been shown to recover quickly, even after decontamination.Reference Schultz, Bjarnsholt and James12 Almatroudi et alReference Almatroudi, Gosbell and Hu8 reported that DSBs regrew within 2 days at 37 °C following 1,000 ppm and 5,000 ppm chlorine treatment. Our study confirmed that observation, with DSBs recovering quickly (<2 days) following the use of 8 of 11 different treatments.
In this study, we have shown that measuring a reduction in viability does not provide enough information to ensure that a surface will be safe after treatment. A significant number of treatments tested (8 of 13) failed to prevent bacteria transfer from treated surfaces, and almost all treatments tested (12 of 13) did not delay biofilm recovery, even though most treatments (9 of 13) were effective at reducing the viability of biofilm. By determining the impact of treatment on bacterial transfer and recovery from DSBs, we have provided additional practical information to manufacturers and end users. Performing these tests in combination would enhance the demonstration of efficacy, providing reassurance that a surface would actually be safe to touch after treatment.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2021.42
Acknowledgments
We thank Filipe Horta (GAMA Healthcare Ltd) for operating the VHP machine.
Financial support
This for this study was provided to Cardiff University by GAMA Healthcare.
Conflicts of interest
K. Ledwoch is partially employed by GAMA Healthcare. All other authors report no conflicts of interest relevant to this article.






